Immune response profiles from humans experimentally exposed to Phlebotomus duboscqi bites
- PMID: 38633260
- PMCID: PMC11021656
- DOI: 10.3389/fimmu.2024.1335307
Immune response profiles from humans experimentally exposed to Phlebotomus duboscqi bites
Abstract
Introduction: Cutaneous leishmaniasis is a neglected vector-borne parasitic disease prevalent in 92 countries with approximately one million new infections annually. Interactions between vector saliva and the human host alter the response to infection and outcome of disease.
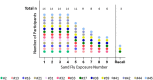
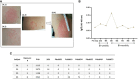
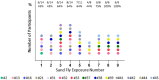
Methods: To characterize the human immunological responses developed against saliva of Phlebotomus duboscqi, a Leishmania major (L. major) vector, we repeatedly exposed the arms of 14 healthy U.S volunteers to uninfected P. duboscqi bites. Blood was collected a week after each exposure and used to assess total IgG antibodies against the proteins of P. duboscqi salivary gland homogenate (SGH) and the levels of IFN-gamma and IL-10 from peripheral blood mononuclear cells (PBMCs) stimulated with SGH or recombinant sand fly proteins. We analyzed skin punch biopsies of the human volunteer arms from the insect bite site and control skin site after multiple P. duboscqi exposures (four volunteers) using immunohistochemical staining.
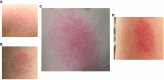
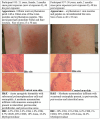
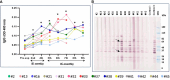
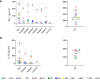
Results: A variety of immediate insect bite skin reactions were observed. Late skin reactions to insect bites were characterized by macular hyperpigmentation and/or erythematous papules. Hematoxylin and eosin staining showed moderate mononuclear skin infiltrate with eosinophils in those challenged recently (within 2 months), eosinophils were not seen in biopsies with recall challenge (6 month post bites). An increase in plasma antigen-specific IgG responses to SGH was observed over time. Western Blot results showed strong plasma reactivity to five P. duboscqi salivary proteins. Importantly, volunteers developed a cellular immunity characterized by the secretion of IFN-gamma upon PBMC stimulation with P. duboscqi SGH and recombinant antigens.
Discussion: Our results demonstrate that humans mounted a local and systemic immune response against P. duboscqi salivary proteins. Specifically, PduM02/SP15-like and PduM73/adenosine deaminase recombinant salivary proteins triggered a Th1 type immune response that might be considered in future development of a potential Leishmania vaccine.
Keywords: P. duboscqi; antigen; immune response; saliva; vaccine.
Copyright © 2024 de Araujo, Abdeladhim, Teixeira, Hummer, Wilkerson, Ressner, Lakhal-Naouar, Ellis, Meneses, Nurmukhambetova, Gomes, Tolbert, Turiansky, Pazgier, Oliveira, Valenzuela, Kamhawi and Aronson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- World Health Organization . Leishmania (2023). Available online at: https://www.who.int/health-topics/leishmaniasis.
-
- Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, et al. . Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. (1998) 188:1941–53. doi: 10.1084/jem.188.10.1941 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

