Promising approaches for simultaneous enhancement of medicinally significant benzylisoquinoline alkaloids in opium poppy
- PMID: 38633462
- PMCID: PMC11022600
- DOI: 10.3389/fpls.2024.1377318
Promising approaches for simultaneous enhancement of medicinally significant benzylisoquinoline alkaloids in opium poppy
Abstract
Benzylisoquinoline alkaloids (BIAs) produced in opium poppy have been evidenced to heal patients suffering from various diseases. They, therefore, hold an integral position in the herbal drug industry. Despite the adoption of several approaches for the large-scale production of BIAs, opium poppy remains the only platform in this purpose. The only disadvantage associated with producing BIAs in the plant is their small quantity. Thus, recruiting strategies that boost their levels is deemed necessary. All the methods which have been employed so far are just able to enhance a maximum of two BIAs. Thus, if these methods are utilized, a sizable amount of time and budget must be spent on the synthesis of all BIAs. Hence, the exploitation of strategies which increase the content of all BIAs at the same time is more commercially effective and time-saving, avoiding the laborious step of resolving the biosynthetic pathway of each compound. Exposure to biotic and abiotic elicitors, development of a synthetic auto-tetraploid, overexpression of a WRKY transcription factor, formation of an artificial metabolon, and suppression of a gene in the shikimate pathway and miRNA are strategies that turn opium poppy into a versatile bioreactor for the concurrent and massive production of BIAs. The last three strategies have never been applied for BIA biosynthetic pathways.
Keywords: CRISPR/Cas9; PsWRKY; autopolyploidy; elicitor; opium poppy; synthetic metabolon.
Copyright © 2024 Aghaali, Naghavi and Zargar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
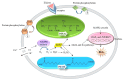
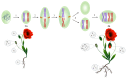
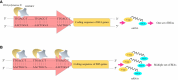
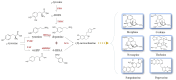
 , L-DOPA;
, L-DOPA;  , dopamine;
, dopamine;  , 4-hydroxyphenylacetaldehyde;
, 4-hydroxyphenylacetaldehyde;  , (S)-norcoclaurine.
, (S)-norcoclaurine.
Similar articles
-
Developing benzylisoquinoline alkaloid-enriched opium poppy via CRISPR-directed genome editing: A review.BMC Plant Biol. 2024 Jul 24;24(1):700. doi: 10.1186/s12870-024-05412-x. BMC Plant Biol. 2024. PMID: 39048937 Free PMC article. Review.
-
Phloem-specific localization of benzylisoquinoline alkaloid metabolism in opium poppy.J Plant Physiol. 2022 Apr;271:153641. doi: 10.1016/j.jplph.2022.153641. Epub 2022 Feb 16. J Plant Physiol. 2022. PMID: 35240512 Review.
-
Benzylisoquinoline alkaloid biosynthesis in opium poppy.Planta. 2014 Jul;240(1):19-32. doi: 10.1007/s00425-014-2056-8. Epub 2014 Mar 27. Planta. 2014. PMID: 24671624 Review.
-
Quantitative 1H NMR metabolomics reveals extensive metabolic reprogramming of primary and secondary metabolism in elicitor-treated opium poppy cell cultures.BMC Plant Biol. 2008 Jan 22;8:5. doi: 10.1186/1471-2229-8-5. BMC Plant Biol. 2008. PMID: 18211706 Free PMC article.
-
Inhibition of phospholipases influences the metabolism of wound-induced benzylisoquinoline alkaloids in Papaver somniferum L.J Plant Physiol. 2018 Apr;223:1-8. doi: 10.1016/j.jplph.2018.01.007. Epub 2018 Jan 31. J Plant Physiol. 2018. PMID: 29433083
Cited by
-
Developing benzylisoquinoline alkaloid-enriched opium poppy via CRISPR-directed genome editing: A review.BMC Plant Biol. 2024 Jul 24;24(1):700. doi: 10.1186/s12870-024-05412-x. BMC Plant Biol. 2024. PMID: 39048937 Free PMC article. Review.
-
Wounding and Phospholipase C Inhibition: Evaluation of the Alkaloid Profiling in Opium Poppy.Plants (Basel). 2025 May 8;14(10):1413. doi: 10.3390/plants14101413. Plants (Basel). 2025. PMID: 40430979 Free PMC article.
References
-
- Agarwal P., Pathak S., Lakhwani D., Gupta P., Asif M. H., Trivedi P. K. (2016). Comparative analysis of transcription factor gene families from Papaver somniferum: identification of regulatory factors involved in benzylisoquinoline alkaloid biosynthesis. Protoplasma 253, 857–871. doi: 10.1007/s00709-015-0848-8 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

