Inhaled volatile anesthetics in the intensive care unit
- PMID: 38633473
- PMCID: PMC11019627
- DOI: 10.5492/wjccm.v13.i1.90746
Inhaled volatile anesthetics in the intensive care unit
Abstract
The discovery and utilization of volatile anesthetics has significantly transformed surgical practices since their inception in the mid-19th century. Recently, a paradigm shift is observed as volatile anesthetics extend beyond traditional confines of the operating theatres, finding diverse applications in intensive care settings. In the dynamic landscape of intensive care, volatile anesthetics emerge as a promising avenue for addressing complex sedation requirements, managing refractory lung pathologies including acute respiratory distress syndrome and status asthmaticus, conditions of high sedative requirements including burns, high opioid or alcohol use and neurological conditions such as status epilepticus. Volatile anesthetics can be administered through either inhaled route via anesthetic machines/devices or through extracorporeal membrane oxygenation circuitry, providing intensivists with multiple options to tailor therapy. Furthermore, their unique pharmacokinetic profiles render them titratable and empower clinicians to individualize management with heightened accuracy, mitigating risks associated with conventional sedation modalities. Despite the amounting enthusiasm for the use of these therapies, barriers to widespread utilization include expanding equipment availability, staff familiarity and training of safe use. This article delves into the realm of applying inhaled volatile anesthetics in the intensive care unit through discussing their pharmacology, administration considerations in intensive care settings, complication considerations, and listing indications and evidence of the use of volatile anesthetics in the critically ill patient population.
Keywords: Anesthesia; Critical care; Mechanical ventilation; Sedation; Sedative; Volatile anesthetics.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no conflicts of interest.
Figures
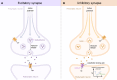
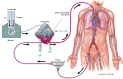

References
-
- Duncum BM. The development of inhalation anaesthesia. London: Royal Soc. of Med. Press. - PubMed
-
- Nunn JF. Nunn's applied respiratory physiology. 6th ed. Edinburgh Philadelphia: Elsevier Butterworth Heinemann.
-
- Bellgardt M, Georgevici AI, Klutzny M, Drees D, Meiser A, Gude P, Vogelsang H, Weber TP, Herzog-Niescery J. Use of MIRUS™ for MAC-driven application of isoflurane, sevoflurane, and desflurane in postoperative ICU patients: a randomized controlled trial. Ann Intensive Care. 2019;9:118. - PMC - PubMed
-
- Coupet R, Schläpfer M, Neff TA, Boucher P, Bailly P, Bellgardt M, Badenes R, Carbonell J, Becher T, Varillon C, Morand D, Blondonnet R, Constantin JM, Pereira B, O'Gara B, Jabaudon M ISCA Study Group. Inhaled Sedation in Patients with COVID-19-Related Acute Respiratory Distress Syndrome: An International Retrospective Study. J Clin Med. 2022;12 - PMC - PubMed
-
- Likhvantsev V, Landoni G, Ermokhina N, Yadgarov M, Berikashvili L, Kadantseva K, Grebenchikov O, Okhinko L, Kuzovlev A. Halogenated anesthetics vs intravenous hypnotics for short and long term sedation in the intensive care unit: A meta-analysis. Med Intensiva (Engl Ed) 2023;47:267–279. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

