Marginal zone lymphoma international prognostic index: a unifying prognostic index for marginal zone lymphomas requiring systemic treatment
- PMID: 38633575
- PMCID: PMC11019091
- DOI: 10.1016/j.eclinm.2024.102592
Marginal zone lymphoma international prognostic index: a unifying prognostic index for marginal zone lymphomas requiring systemic treatment
Abstract
Background: Marginal zone lymphomas (MZL), comprised of three unique but related subtypes, lack a unifying prognostic score applicable to all the patients in need for systemic chemotherapy and/or immunotherapy.
Methods: Patients from the prospective NF10 study (NCT02904577) with newly diagnosed MZL and receiving frontline systemic therapy at diagnosis or after observation were used to train a prognostic model. The primary endpoint was progression-free survival (PFS) from start of treatment. The model was externally validated in a pooled analysis of two independent cohorts from the University of Iowa and Mayo Clinic Molecular Epidemiology Resource and the University of Miami.
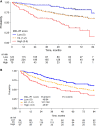
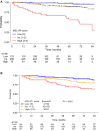
Findings: We identified 501 eligible patients. After multivariable modeling, lactate dehydrogenase (LDH) above upper normal limit, hemoglobin <12 g/dL, absolute lymphocyte count <1 × 109/L, platelets <100 × 109/L, and MZL subtype (nodal or disseminated) were independently associated with inferior PFS. The proposed MZL International Prognostic index (MZL-IPI) combined these 5 factors, and we defined low (LRG, 0 factors, 27%), intermediate (IRG, 1-2 factors, 57%) and high (HRG, 3+ factors, 16%) risk groups with 5-y PFS of 85%, 66%, and 37%, respectively (c-Harrell = 0.64). Compared to the LRG, the IRG (Hazard Ratio [HR] = 2.30, 95% CI 1.39-3.80) and HRG (HR = 5.41, 95% CI 3.12-9.38) had inferior PFS. Applying the MZL-IPI to the pooled US cohort (N = 353), 94 (27%), 192 (54%), and 67 (19%) patients were classified as LRG, IRG, and HRG, respectively, and the model was validated for PFS (log-rank test p = 0.0018; c-Harrell = 0.578, 95% CI 0.54-0.62). The MZL-IPI was also prognostic for OS in both the training and the external validation sets.
Interpretation: MZL-IPI is a new prognostic score for use in all patients with MZL considered for systemic treatment.
Funding: The MER was supported by P50 CA97274 and U01 CA195568.
Keywords: Marginal zone lymphoma; Prognosis.
© 2024 The Author(s).
Conflict of interest statement
LA: Grants or contracts from any entity: My First AIRC grant n. 11,415 2012–2014; Investigator Grant AIRC (2018–2022); Honoraria: EUSA Pharma, Novartis. Participation on a Data Safety Monitoring Board or Advisory Board Roche, Janssen-Cilag, Verastem, Incyte, EUSA Pharma, Celgene/Bristol Myers Squibb, Kite/Gilead, ADC Therapeutics, Novartis; Support for attending meetings and/or travel: Roche. CB: Grants or contracts from any entity: INSERM, AvieSan ITMO Cancer, LYSA/ELI: Bertrand Coiffier Prize Institut Servier; Consulting fees: Currety; Support for attending meetings and/or travel: Mayo Clinic. JPA: Grants or contracts from any entity: Lymphoma Research Foundation, US Department of Defense; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: ADC Therapeutics, Regeneron, Genentech. MM: Support for attending meetings and/or travel: Janssen. MJM: Grants or contracts from any entity: BMS, Roche, GenMab; Consulting fees: BMS; Participation on a Data Safety Monitoring Board or Advisory Board: AstraZeneca. CV: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Janssen, Lilly, Novartis, Gilead, Takeda, Kyowa-Kirin, Roche, Astra Zeneca, Beigene, Gentili. BKL: Grants or contracts from any entity: Roche/Genentech, Seattle Genetics, Genmab, AstraZeneca. OA: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Roche, Janssen, Beigene, Ely Lilli, Amgen, Sanofi. AP: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Roche, Msd, Pfizer, Sandoz, Takeda, Gilead, Bms, Janssen, Beigene; Participation on a Data Safety Monitoring Board or Advisory Board: Takeda, Roche. TMH: All support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.): Lymphoma SPORE NCI CA 97274; Participation on a Data Safety Monitoring Board or Advisory Board: Seagen, Eli Lilly & Co. LM: Other financial or non-financial interests: Scientific consultant for Sandoz spa, 2022–2023, free of fee. JRC: All support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.): National Cancer Institute, Grants P50 CA97274 and U01 CA195568 (to Mayo); Grants or contracts from any entity: BMS, Genentech, and Genmab; Participation on a Data Safety Monitoring Board or Advisory Board and SMC member: Protagonist Therapeutics. SL: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Roche, Novartis, Incyte, BMS, Kite, Regeneron, Abbvie, Genmab, Sobi, Beigene; Support for attending meetings and/or travel: Roche, Beigene, Regeneron. NF, MEN, VT, SF, SR, AT, RM, AK, RM, MD, EC, FR, ALF, MDT, MS, AJMF, CS, EP, SH, IMR, ISL: no COI.
Figures
References
-
- Rossi D., Bertoni F., Zucca E. Marginal-zone lymphomas. N Engl J Med. 2022;386:568–581. - PubMed
-
- Zucca E., Arcaini L., Buske C., et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:17–29. - PubMed
-
- Iannitto E., Bellei M., Amorim S., et al. Efficacy of bendamustine and rituximab in splenic marginal zone lymphoma: results from the phase II BRISMA/IELSG36 study. Br J Haematol. 2018;183:755–765. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

