Differential Susceptibility to Propofol and Ketamine in Primary Cultures of Young and Senesced Astrocytes
- PMID: 38633620
- PMCID: PMC11023735
- DOI: 10.1155/2024/8876548
Differential Susceptibility to Propofol and Ketamine in Primary Cultures of Young and Senesced Astrocytes
Abstract
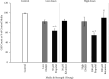
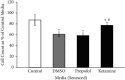
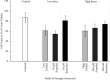
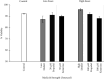
The adverse effects of general anesthesia on the long-term cognition of young children and senior adults have become of concern in recent years. Previously, mechanistic and pathogenic investigations focused on neurons, and little is known about the effect of commonly used intravenous anesthetics such as propofol and ketamine on astrocytes. Recently, astrocyte dysfunction has been implicated in a wide range of age-related brain diseases. In this study, we examined the survival and viability of both young and senescent astrocytes in culture after adding propofol and ketamine to the media at varying strengths. Oxidative stimulus was applied to commercially available fetal cell lines of human astrocytes in vitro to induce morphological changes in cellular senescence. Our results indicate that propofol reduces the survival of young astrocytes as compared to controls, as well as to ketamine. These effects were seen in comparisons of total cell count and at both high and low dose concentrations. High doses of propofol also significantly reduced cell viability compared to those exposed to baseline controls and ketamine. Senescent astrocytes, on the other hand, demonstrated cell count reductions as compared to baseline controls and ketamine when exposed to either DMSO or propofol. The data show differential susceptibility of young astrocytes to propofol than to ketamine. The observed cell count reduction may be related to the adverse effects of propofol on mitochondrial function and free radical production, as described in previous studies. We speculate that ketamine may have a more favorable safety profile in infants and young children.
Copyright © 2024 Liang Huang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

