Research progress on optimization of in vitro isolation, cultivation and preservation methods of dental pulp stem cells for clinical application
- PMID: 38633667
- PMCID: PMC11021638
- DOI: 10.3389/fbioe.2024.1305614
Research progress on optimization of in vitro isolation, cultivation and preservation methods of dental pulp stem cells for clinical application
Abstract
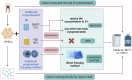
Due to high proliferative capacity, multipotent differentiation, immunomodulatory abilities, and lack of ethical concerns, dental pulp stem cells (DPSCs) are promising candidates for clinical application. Currently, clinical research on DPSCs is in its early stages. The reason for the failure to obtain clinically effective results may be problems with the production process of DPSCs. Due to the different preparation methods and reagent formulations of DPSCs, cell characteristics may be affected and lead to inconsistent experimental results. Preparation of clinical-grade DPSCs is far from ready. To achieve clinical application, it is essential to transit the manufacturing of stem cells from laboratory grade to clinical grade. This review compares and analyzes experimental data on optimizing the preparation methods of DPSCs from extraction to resuscitation, including research articles, invention patents and clinical trials. The advantages and disadvantages of various methods and potential clinical applications are discussed, and factors that could improve the quality of DPSCs for clinical application are proposed. The aim is to summarize the current manufacture of DPSCs in the establishment of a standardized, reliable, safe, and economic method for future preparation of clinical-grade cell products.
Keywords: 3D cell culture; clinical application; cryopreservation; dental pulp stem cells; serum-free culture; stem cell property.
Copyright © 2024 Wang, Li, Wu, Xing, Fu, Wang and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

