IKZF1plus is a frequent biomarker of adverse prognosis in Mexican pediatric patients with B-acute lymphoblastic leukemia
- PMID: 38634053
- PMCID: PMC11022689
- DOI: 10.3389/fonc.2024.1337954
IKZF1plus is a frequent biomarker of adverse prognosis in Mexican pediatric patients with B-acute lymphoblastic leukemia
Abstract
Background: Recurrent genetic alterations contributing to leukemogenesis have been identified in pediatric B-cell Acute Lymphoblastic Leukemia (B-ALL), and some are useful for refining classification, prognosis, and treatment selection. IKZF1plus is a complex biomarker associated with a poor prognosis. It is characterized by IKZF1 deletion coexisting with PAX5, CDKN2A/2B, or PAR1 region deletions. The mutational spectrum and clinical impact of these alterations have scarcely been explored in Mexican pediatric patients with B-ALL. Here, we report the frequency of the IKZF1plus profile and the mutational spectrum of IKZF1, PAX5, CDKN2A/2B, and ERG genes and evaluate their impact on overall survival (OS) in a group of patients with B-ALL.
Methods: A total of 206 pediatric patients with de novo B-ALL were included. DNA was obtained from bone marrow samples at diagnosis before treatment initiation. A custom-designed next-generation sequencing panel was used for mutational analysis. Kaplan-Meier analysis was used for OS estimation.
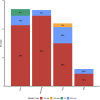
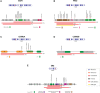
Results: We identified the IKZF1plus profile in 21.8% of patients, which was higher than that previously reported in other studies. A significantly older age (p=0.04), a trend toward high-risk stratification (p=0.06), and a decrease in 5-year Overall Survival (OS) (p=0.009) were observed, although heterogeneous treatment protocols in our cohort would have impacted OS. A mutation frequency higher than that reported was found for IKZF1 (35.9%) and CDKN2A/2B (35.9%) but lower for PAX5 (26.6%). IKZF1MUT group was older at diagnosis (p=0.0002), and most of them were classified as high-risk (73.8%, p=0.02), while patients with CDKN2A/2BMUT had a higher leukocyte count (p=0.01) and a tendency toward a higher percentage of blasts (98.6%, >50% blasts, p=0.05) than the non-mutated patients. A decrease in OS was found in IKZF1MUT and CDKN2A/2BMUT patients, but the significance was lost after IKZF1plus was removed.
Discussion: Our findings demonstrated that Mexican patients with B-ALL have a higher prevalence of genetic markers associated with poor outcomes. Incorporating genomic methodologies into the diagnostic process, a significant unmet need in low- and mid-income countries, will allow a comprehensive identification of relevant alterations, improving disease classification, treatment selection, and the general outcome.
Keywords: CDKN2A/2B gene mutation; IKZF1 gene mutation; IKZF1plus; PAR1 deletions; PAX5 gene mutation; overall survival; pediatric B-ALL.
Copyright © 2024 Garcia-Solorio, Núñez-Enriquez, Jiménez-Olivares, Flores-Lujano, Flores-Espino, Molina-Garay, Cervera, Casique-Aguirre, Peñaloza-Gonzalez, Baños-Lara, García-Soto, Galván-Díaz, Olaya-Vargas, Aguilar, Mata-Rocha, Garrido-Hernández, Solís-Poblano, Luna-Silva, Cano-Cuapio, Aristil-Chery, Herrera-Quezada, Carrillo-Sanchez, Muñoz-Rivas, Flores-Lagunes, Mendoza-Caamal, Villegas-Torres, González-Osnaya, Jiménez-Hernández, Torres-Nava, Martín-Trejo, Gutiérrez-Rivera, Espinosa-Elizondo, Merino-Pasaye, Pérez-Saldívar, Jiménez-Morales, Curiel-Quesada, Rosas-Vargas, Mejía-Arangure and Alaez-Verson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer, AM, declared a shared affiliation with the author RE-E to the handling editor at the time of review.
Figures

References
-
- Steeghs EMP, Boer JM, Hoogkamer AQ, Boeree A, de Haas V, de Groot-Kruseman HA, et al. Copy number alterations in B-cell development genes, drug resistance, and clinical outcome in pediatric B-cell precursor acute lymphoblastic leukemia. Sci Rep. (2019) 9:4634. doi: 10.1038/s41598-019-41078-4 - DOI - PMC - PubMed
-
- Braun M, Pastorczak A, Sędek Ł, Taha J, Madzio J, Jatczak-Pawlik I, et al. Prognostic significance of IKZF1 deletions and IKZF1plus profile in children with B-cell precursor acute lymphoblastic leukemia treated according to the ALL-IC BFM 2009 protocol. Hematol Oncol. (2022) 40:430–41. doi: 10.1002/hon.2973 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

