Commensal microbiota regulate aldosterone
- PMID: 38634136
- PMCID: PMC11381011
- DOI: 10.1152/ajprenal.00051.2024
Commensal microbiota regulate aldosterone
Abstract
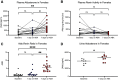
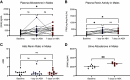
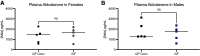
The gut microbiome regulates many important host physiological processes associated with cardiovascular health and disease; however, the impact of the gut microbiome on aldosterone is unclear. Investigating whether gut microbiota regulate aldosterone can offer novel insights into how the microbiome affects blood pressure. In this study, we aimed to determine whether gut microbiota regulate host aldosterone. We used enzyme-linked immunosorbent assays (ELISAs) to assess plasma aldosterone and plasma renin activity (PRA) in female and male mice in which gut microbiota are intact, suppressed, or absent. In addition, we examined urinary aldosterone. Our findings demonstrated that when the gut microbiota is suppressed following antibiotic treatment, there is an increase in plasma and urinary aldosterone in both female and male mice. In contrast, an increase in PRA is seen only in males. We also found that when gut microbiota are absent (germ-free mice), plasma aldosterone is significantly increased compared with conventional animals (in both females and males), but PRA is not. Understanding how gut microbiota influence aldosterone levels could provide valuable insights into the development and treatment of hypertension and/or primary aldosteronism. This knowledge may open new avenues for therapeutic interventions, such as probiotics or dietary modifications to help regulate blood pressure via microbiota-based changes to aldosterone.NEW & NOTEWORTHY We explore the role of the gut microbiome in regulating aldosterone, a hormone closely linked to blood pressure and cardiovascular disease. Despite the recognized importance of the gut microbiome in host physiology, the relationship with circulating aldosterone remains largely unexplored. We demonstrate that suppression of gut microbiota leads to increased levels of plasma and urinary aldosterone. These findings underscore the potential of the gut microbiota to influence aldosterone regulation, suggesting new possibilities for treating hypertension.
Keywords: RAAS; aldosterone; gut microbiome; renal physiology.
Conflict of interest statement
Jennifer L. Pluznick is an editor of the
Figures


References
-
- Nakai M, Ribeiro RV, Stevens BR, Gill P, Muralitharan RR, Yiallourou S, Muir J, Carrington M, Head GA, Kaye DM, Marques FZ. Essential hypertension is associated with changes in gut microbial metabolic pathways: a multisite analysis of ambulatory blood pressure. Hypertension 78: 804–815, 2021. doi: 10.1161/HYPERTENSIONAHA.121.17288. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

