Are asymptomatic carriers of OTC deficiency always asymptomatic? A multicentric retrospective study of risk using the UCDC longitudinal study database
- PMID: 38634223
- PMCID: PMC11024633
- DOI: 10.1002/mgg3.2443
Are asymptomatic carriers of OTC deficiency always asymptomatic? A multicentric retrospective study of risk using the UCDC longitudinal study database
Abstract
Background: Ornithine transcarbamylase deficiency (OTCD) due to an X-linked OTC mutation, is responsible for moderate to severe hyperammonemia (HA) with substantial morbidity and mortality. About 80% of females with OTCD remain apparently "asymptomatic" with limited studies of their clinical characteristics and long-term health vulnerabilities. Multimodal neuroimaging studies and executive function testing have shown that asymptomatic females exhibit limitations when stressed to perform at higher cognitive load and had reduced activation of the prefrontal cortex. This retrospective study aims to improve understanding of factors that might predict development of defined complications and serious illness in apparent asymptomatic females. A proband and her daughter are presented to highlight the utility of multimodal neuroimaging studies and to underscore that asymptomatic females with OTCD are not always asymptomatic.
Methods: We review data from 302 heterozygote females with OTCD enrolled in the Urea Cycle Disorders Consortium (UCDC) longitudinal natural history database. We apply multiple neuroimaging modalities in the workup of a proband and her daughter.
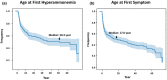
Results: Among the females in the database, 143 were noted as symptomatic at baseline (Sym). We focused on females who were asymptomatic (Asx, n = 111) and those who were asymptomatic initially upon enrollment in study but who later became symptomatic sometime during follow-up (Asx/Sym, n = 22). The majority of Asx (86%) and Asx/Sym (75%) subjects did not restrict protein at baseline, and ~38% of Asx and 33% of Asx/Sym subjects suffered from mild to severe neuropsychiatric conditions such as mood disorder and sleep problems. The risk of mild to severe HA sometime later in life for the Asx and Asx/Sym subjects as a combined group was ~4% (5/133), with ammonia ranging from 77 to 470 μM and at least half (2/4) of subjects requiring hospital admission and nitrogen scavenger therapy. For this combined group, the median age of first HA crisis was 50 years, whereas the median age of first symptom which included neuropsychiatric and/or behavioral symptoms was 17 years. The multimodal neuroimaging studies in female heterozygotes with OTCD also underscore that asymptomatic female heterozygotes with OTCD (e.g., proband) are not always asymptomatic.
Conclusions: Analysis of Asx and Asx/Sym females with OTCD in this study suggests that future evidence-based management guidelines and/or a clinical risk score calculator for this cohort could be useful management tools to reduce morbidity and improve long-term quality of life.
Keywords: Urea Cycle Disorders Consortium; diffusion tensor imaging; functional MRI; functional near‐infrared spectroscopy; hyperammonemia; magnetic resonance spectroscopy; ornithine transcarbamylase deficiency; prefrontal cortex; urea cycle disorder.
© 2024 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors report no conflict of interest.
Figures

References
-
- Brassier, A. , Gobin, S. , Arnoux, J. B. , Valayannopoulos, V. , Habarou, F. , Kossorotoff, M. , Servais, A. , Barbier, V. , Dubois, S. , Touati, G. , Barouki, R. , Lesage, F. , Dupic, L. , Bonnefont, J. P. , Ottolenghi, C. , & De Lonlay, P. (2015). Long‐term outcomes in Ornithine Transcarbamylase deficiency: A series of 90 patients. Orphanet Journal of Rare Diseases, 10, 58. 10.1186/s13023-015-0266-1 - DOI - PMC - PubMed
-
- Brusilow, S. W. , & Maestri, N. E. (1996). Urea cycle disorders: Diagnosis, pathophysiology, and therapy. Adv Pediatr, 43, 127–170. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

