Reducing neonatal Fc receptor binding enhances clearance and brain-to-blood ratio of TfR-delivered bispecific amyloid-β antibody
- PMID: 38634473
- PMCID: PMC11028011
- DOI: 10.1080/19420862.2024.2339337
Reducing neonatal Fc receptor binding enhances clearance and brain-to-blood ratio of TfR-delivered bispecific amyloid-β antibody
Abstract
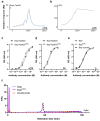
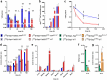
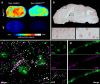
Recent development of amyloid-β (Aβ)-targeted immunotherapies for Alzheimer's disease (AD) have highlighted the need for accurate diagnostic methods. Antibody-based positron emission tomography (PET) ligands are well suited for this purpose as they can be directed toward the same target as the therapeutic antibody. Bispecific, brain-penetrating antibodies can achieve sufficient brain concentrations, but their slow blood clearance remains a challenge, since it prolongs the time required to achieve a target-specific PET signal. Here, two antibodies were designed based on the Aβ antibody bapineuzumab (Bapi) - one monospecific IgG (Bapi) and one bispecific antibody with an antigen binding fragment (Fab) of the transferrin receptor (TfR) antibody 8D3 fused to one of the heavy chains (Bapi-Fab8D3) for active, TfR-mediated transport into the brain. A variant of each antibody was designed to harbor a mutation to the neonatal Fc receptor (FcRn) binding domain, to increase clearance. Blood and brain pharmacokinetics of radiolabeled antibodies were studied in wildtype (WT) and AD mice (AppNL-G-F). The FcRn mutation substantially reduced blood half-life of both Bapi and Bapi-Fab8D3. Bapi-Fab8D3 showed high brain uptake and the brain-to-blood ratio of its FcRn mutated form was significantly higher in AppNL-G-F mice than in WT mice 12 h after injection and increased further up to 168 h. Ex vivo autoradiography showed specific antibody retention in areas with abundant Aβ pathology. Taken together, these results suggest that reducing FcRn binding of a full-sized bispecific antibody increases the systemic elimination and could thereby drastically reduce the time from injection to in vivo imaging.
Keywords: Alzheimer’s disease (AD); amyloid-β (aβ); bispecific antibody; blood-brain barrier (BBB); neonatal Fc receptor (FcRn); receptor mediated transcytosis (RMT).
Conflict of interest statement
Ken G. Andersson is an employee of BioArctic AB, Sweden. The other authors have no conflicts of interest to declare.
Figures


References
-
- FDA . FDA grants accelerated approval for Alzheimer’s disease treatment. 2023. [accessed 2023 Jan 6 https://www.fda.gov/news-events/press-announcements/fda-grants-accelerat....
-
- Cavazzoni P. (FDA C. for D. E. and R. FDA’s decision to approve new treatment for Alzheimer’s disease. Fda’s decision to approve new treatment for Alzheimer’s disease 2021. https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
