Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A Systematic Review and Meta-Analysis With Insights for Periprocedural Management
- PMID: 38634551
- PMCID: PMC11150091
- DOI: 10.14309/ajg.0000000000002820
Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A Systematic Review and Meta-Analysis With Insights for Periprocedural Management
Abstract
Introduction: Divergent recommendations for periprocedural management of glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1 RA) medications rely on limited evidence. We performed a systematic review and meta-analysis to provide quantitative measures of gastric emptying relevant to mechanisms of weight loss and to periprocedural management of GLP-1 RA. We hypothesized that the magnitude of gastric emptying delay would be low and of limited clinical significance to procedural sedation risks.
Methods: A protocolized search identified studies on GLP-1 RA that quantified gastric emptying measures. Pooled estimates using random effects were presented as a weighted mean difference with 95% confidence intervals (CIs). Univariate meta-regression was performed to assess the influence of GLP-1 RA type, short-acting vs long-acting mechanism of action, and duration of treatment on gastric emptying.
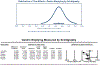
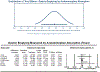
Results: Fifteen studies met the inclusion criteria. Five studies (n = 247) utilized gastric emptying scintigraphy. Mean T 1/2 was 138.4 minutes (95% CI 74.5-202.3) for GLP-1 RA vs 95.0 minutes (95% CI 54.9-135.0) for placebo, with a pooled mean difference of 36.0 minutes (95% CI 17.0-55.0, P < 0.01, I2 = 79.4%). Ten studies (n = 411) utilized the acetaminophen absorption test, with no significant delay in gastric emptying measured by T max , area under the curve (AUC) 4hr , and AUC 5hr with GLP-1 RA ( P > 0.05). On meta-regression, the type of GLP-1 RA, mechanism of action, and treatment duration did not impact gastric emptying ( P > 0.05).
Discussion: While a gastric emptying delay of ∼36 minutes is quantifiable on GLP-1 RA medications, it is of limited magnitude relative to standard periprocedural fasting periods. There were no substantial differences in gastric emptying on modalities reflective of liquid emptying (acetaminophen absorption test), particularly at time points relevant to periprocedural care.
Copyright © 2024 by The American College of Gastroenterology.
Conflict of interest statement
Potential Conflicts of Interest:
Walter Chan served on the advisory board for Phathom Pharmaceuticals, Sanofi Pharmaceuticals, and Regeneron Pharmaceuticals. No other authors have potential conflicts of interest to disclose.
Figures

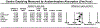
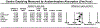


References
-
- Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. Oct 2021;9(10):653–662. doi:10.1016/S2213-8587(21)00203-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

