Retrograde Solubility of Methylammonium Lead Iodide in γ-Butyrolactone Does Not Enhance the Uniformity of Continuously Coated Films
- PMID: 38634602
- PMCID: PMC11197085
- DOI: 10.1021/acs.langmuir.3c03979
Retrograde Solubility of Methylammonium Lead Iodide in γ-Butyrolactone Does Not Enhance the Uniformity of Continuously Coated Films
Abstract
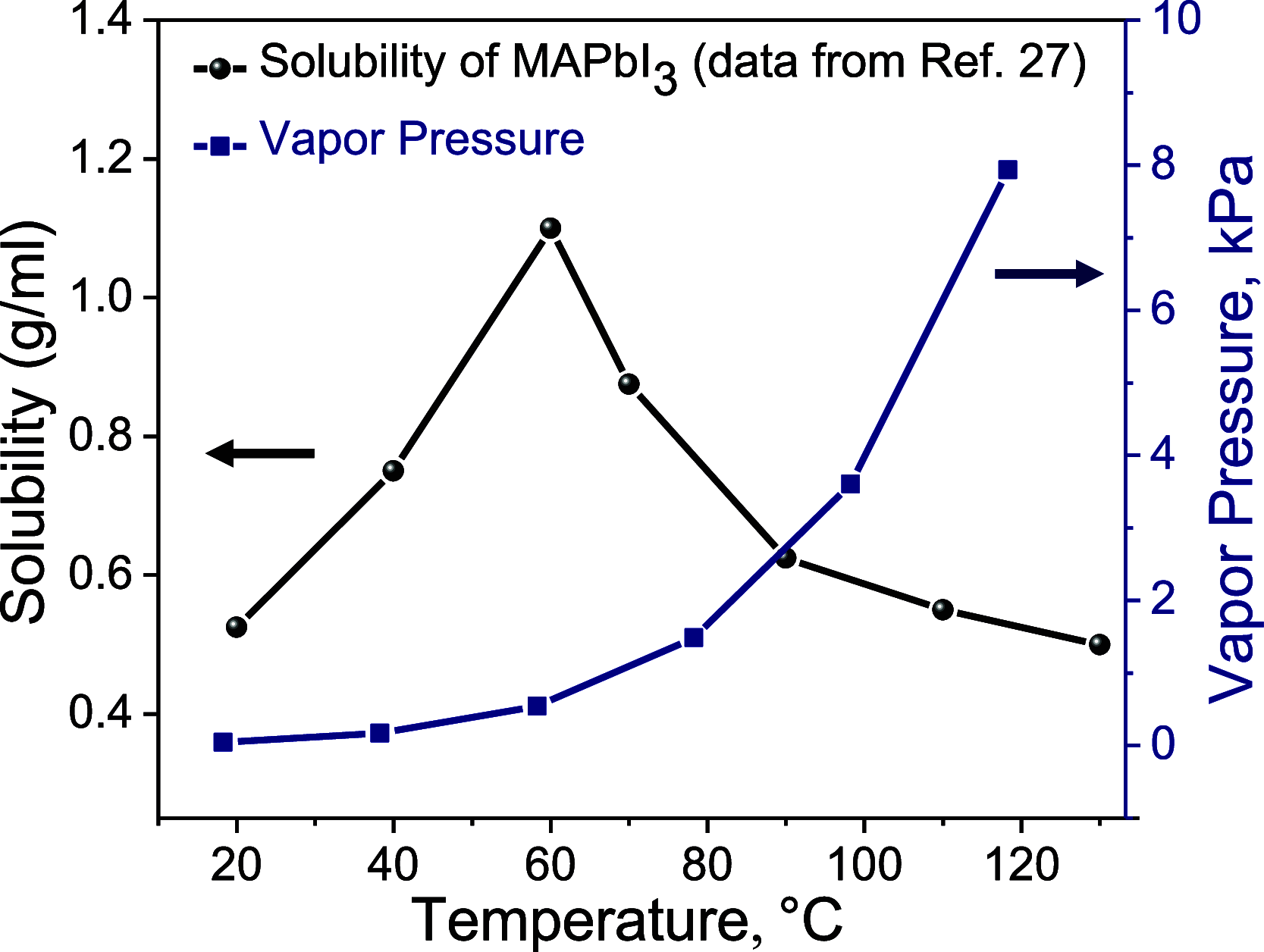
Halide perovskite thin films can be the centerpiece of high-performance solar cells, light-emitting diodes, and other optoelectronic devices if the films are of high uniformity and relatively free of pinholes and other defects. A common strategy to form dense films from solution has been to generate a high density of nuclei by rapidly increasing supersaturation, for example, by timely application of an antisolvent or forced convection. In this work, we examine the role of retrograde solubility, wherein solubility decreases with increasing temperature, as a means of increasing the nucleation density and film coverage of slot-die-coated methylammonium lead iodide (MAPbI3) from γ-butyrolactone (GBL) solution. Coverage was investigated as a function of the substrate temperature and the presence and temperature of an air knife. Results were considered within the framework of the dimensionless modified Biot number, which quantifies the interplay between evaporation and horizontal diffusion. Moderate temperatures and a heated air knife improved film coverage and morphology by enhanced nucleation up to ∼80 °C. However, despite the dense nucleation enabled by retrograde solubility, slow evaporation as a result of the low vapor pressure of GBL, combined with Ostwald ripening at high temperatures, prevented the deposition of void-free, device-quality films. This work has provided a more detailed understanding of the interplay between perovskite processing, solvent parameters, and film morphology and ultimately indicates the obstacles to forming dense, uniform films from solvents with high boiling points even in the presence of rapid nucleation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- National Renewable Energy Laboratory (NREL) . Best Research-Cell Efficiencies Chart; NREL: Golden, CO, 2024; https://www.nrel.gov/pv/cell-efficiency.html.
LinkOut - more resources
Full Text Sources

