Enhanced Vascular-like Network Formation of Encapsulated HUVECs and ADSCs Coculture in Growth Factors Conjugated GelMA Hydrogels
- PMID: 38634810
- PMCID: PMC11094682
- DOI: 10.1021/acsbiomaterials.4c00465
Enhanced Vascular-like Network Formation of Encapsulated HUVECs and ADSCs Coculture in Growth Factors Conjugated GelMA Hydrogels
Abstract
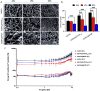
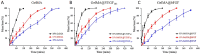
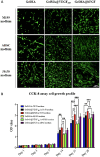
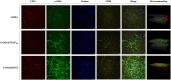
Tissue engineering primarily aimed to alleviate the insufficiency of organ donations worldwide. Nonetheless, the survival of the engineered tissue is often compromised due to the complexity of the natural organ architectures, especially the vascular system inside the organ, which allows food-waste transfer. Thus, vascularization within the engineered tissue is of paramount importance. A critical aspect of this endeavor is the ability to replicate the intricacies of the extracellular matrix and promote the formation of functional vascular networks within engineered constructs. In this study, human adipose-derived stem cells (hADSCs) and human umbilical vein endothelial cells (HUVECs) were cocultured in different types of gelatin methacrylate (GelMA). In brief, pro-angiogenic signaling growth factors (GFs), vascular endothelial growth factor (VEGF165) and basic fibroblast growth factor (bFGF), were conjugated onto GelMA via an EDC/NHS coupling reaction. The GelMA hydrogels conjugated with VEGF165 (GelMA@VEGF165) and bFGF (GelMA@bFGF) showed marginal changes in the chemical and physical characteristics of the GelMA hydrogels. Moreover, the conjugation of these growth factors demonstrated improved cell viability and cell proliferation within the hydrogel construct. Additionally, vascular-like network formation was observed predominantly on GelMA@GrowthFactor (GelMA@GF) hydrogels, particularly on GelMA@bFGF. This study suggests that growth factor-conjugated GelMA hydrogels would be a promising biomaterial for 3D vascular tissue engineering.
Keywords: bFGF; gelMA; hADSCs; hUVECs; vEGF; vascular formation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Gelatin-based micro-hydrogel carrying genetically engineered human endothelial cells for neovascularization.Acta Biomater. 2019 Sep 1;95:285-296. doi: 10.1016/j.actbio.2019.01.057. Epub 2019 Jan 31. Acta Biomater. 2019. PMID: 30710712
-
Coculture of mesenchymal stem cells and endothelial cells enhances host tissue integration and epidermis maturation through AKT activation in gelatin methacryloyl hydrogel-based skin model.Acta Biomater. 2017 Sep 1;59:317-326. doi: 10.1016/j.actbio.2017.07.001. Epub 2017 Jul 3. Acta Biomater. 2017. PMID: 28684336
-
3D-Bioprinted GelMA Scaffold with ASCs and HUVECs for Engineering Vascularized Adipose Tissue.ACS Appl Bio Mater. 2024 Jan 15;7(1):406-415. doi: 10.1021/acsabm.3c00964. Epub 2023 Dec 26. ACS Appl Bio Mater. 2024. PMID: 38148527
-
Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels.Biomaterials. 2015 Dec;73:254-71. doi: 10.1016/j.biomaterials.2015.08.045. Epub 2015 Aug 28. Biomaterials. 2015. PMID: 26414409 Free PMC article. Review.
-
Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: an Effective Strategy for Tissue Engineering.Stem Cell Rev Rep. 2019 Oct;15(5):664-679. doi: 10.1007/s12015-019-09893-4. Stem Cell Rev Rep. 2019. PMID: 31154619 Review.
Cited by
-
Advancing Bioprinting Technology Utilizing Portable Bioprinters: From Various Device Designs to Dental Applications.Ann Biomed Eng. 2025 Jul 21. doi: 10.1007/s10439-025-03789-w. Online ahead of print. Ann Biomed Eng. 2025. PMID: 40690120 Review.
-
Cell-Free Fat Extract (Ceffe) Combined with GelMA Hydrogel to Improve the Survival Rate of Random Skin Flaps in Mice.ACS Biomater Sci Eng. 2025 Jul 14;11(7):4177-4192. doi: 10.1021/acsbiomaterials.4c02259. Epub 2025 Jun 2. ACS Biomater Sci Eng. 2025. PMID: 40457529 Free PMC article.
References
-
- Malda J.; Woodfield T. B. F.; van der Vloodt F.; Kooy F. K.; Martens D. E.; Tramper J.; Blitterswijk C. A. V.; Riesle J. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 2004, 25 (26), 5773–5780. 10.1016/j.biomaterials.2004.01.028. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
