Formation of Calprotectin Inhibits Amyloid Aggregation of S100A8 and S100A9 Proteins
- PMID: 38634811
- PMCID: PMC11066842
- DOI: 10.1021/acschemneuro.4c00093
Formation of Calprotectin Inhibits Amyloid Aggregation of S100A8 and S100A9 Proteins
Abstract
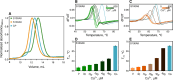
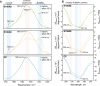
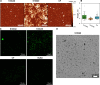
Calcium-binding S100A8 and S100A9 proteins play a significant role in various disorders due to their pro-inflammatory functions. Substantially, they are also relevant in neurodegenerative disorders via the delivery of signals for the immune response. However, at the same time, they can aggregate and accelerate the progression of diseases. Natively, S100A8 and S100A9 exist as homo- and heterodimers, but upon aggregation, they form amyloid-like oligomers, fibrils, or amorphous aggregates. In this study, we aimed to elucidate the aggregation propensities of S100A8, S100A9, and their heterodimer calprotectin by investigating aggregation kinetics, secondary structures, and morphologies of the aggregates. For the first time, we followed the in vitro aggregation of S100A8, which formed spherical aggregates, unlike the fibrillar structures of S100A9 under the same conditions. The aggregates were sensitive to amyloid-specific ThT and ThS dyes and had a secondary structure composed of β-sheets. Similarly to S100A9, S100A8 protein was stabilized by calcium ions, resulting in aggregation inhibition. Finally, the formation of S100A8 and S100A9 heterodimers stabilized the proteins in the absence of calcium ions and prevented their aggregation.
Keywords: S100; aggregation; amyloid; inflammation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Calcium-Dependent S100A8 Amyloid Fibril Formation via S100A1-Mediated Transient Interaction.ACS Chem Neurosci. 2025 Jul 16;16(14):2592-2601. doi: 10.1021/acschemneuro.5c00086. Epub 2025 Jun 25. ACS Chem Neurosci. 2025. PMID: 40560667 Free PMC article.
-
Pro-inflammatory S100A8 and S100A9 proteins: self-assembly into multifunctional native and amyloid complexes.Int J Mol Sci. 2012;13(3):2893-2917. doi: 10.3390/ijms13032893. Epub 2012 Mar 5. Int J Mol Sci. 2012. PMID: 22489132 Free PMC article. Review.
-
Aggregation of human S100A8 and S100A9 amyloidogenic proteins perturbs proteostasis in a yeast model.PLoS One. 2013;8(3):e58218. doi: 10.1371/journal.pone.0058218. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483999 Free PMC article.
-
The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins.J Mol Biol. 2007 Jul 27;370(5):887-98. doi: 10.1016/j.jmb.2007.04.065. Epub 2007 May 3. J Mol Biol. 2007. PMID: 17553524
-
Calprotectin and the Initiation and Progression of Head and Neck Cancer.J Dent Res. 2018 Jun;97(6):674-682. doi: 10.1177/0022034518756330. Epub 2018 Feb 14. J Dent Res. 2018. PMID: 29443623 Free PMC article. Review.
Cited by
-
Calcium-Dependent S100A8 Amyloid Fibril Formation via S100A1-Mediated Transient Interaction.ACS Chem Neurosci. 2025 Jul 16;16(14):2592-2601. doi: 10.1021/acschemneuro.5c00086. Epub 2025 Jun 25. ACS Chem Neurosci. 2025. PMID: 40560667 Free PMC article.
-
Heterotypic Droplet Formation by Pro-Inflammatory S100A9 and Neurodegenerative Disease-Related α-Synuclein.Biomacromolecules. 2025 Jun 9;26(6):3525-3537. doi: 10.1021/acs.biomac.5c00130. Epub 2025 May 15. Biomacromolecules. 2025. PMID: 40370127 Free PMC article.
-
Binding of Pro-Inflammatory Proteins S100A8 or S100A9 to Amyloid-β Peptide Suppresses Its Fibrillation.Biomolecules. 2025 Mar 17;15(3):431. doi: 10.3390/biom15030431. Biomolecules. 2025. PMID: 40149967 Free PMC article.
-
Pro-inflammatory S100A8 Protein Exhibits a Detergent-like Effect on Anionic Lipid Bilayers, as Imaged by High-Speed AFM.ACS Appl Mater Interfaces. 2025 Jan 8;17(1):2635-2647. doi: 10.1021/acsami.4c18749. Epub 2024 Dec 26. ACS Appl Mater Interfaces. 2025. PMID: 39723944 Free PMC article.
-
Changing expression system alters oligomerization and proinflammatory activity of recombinant human S100A9.bioRxiv [Preprint]. 2024 Aug 14:2024.08.14.608001. doi: 10.1101/2024.08.14.608001. bioRxiv. 2024. PMID: 39185185 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

