Global real-world experiences with pembrolizumab in advanced urothelial carcinoma after platinum-based chemotherapy: the ARON-2 study
- PMID: 38634928
- PMCID: PMC11026312
- DOI: 10.1007/s00262-024-03682-w
Global real-world experiences with pembrolizumab in advanced urothelial carcinoma after platinum-based chemotherapy: the ARON-2 study
Abstract
Background: Immune checkpoint inhibitors have changed previous treatment paradigm of advanced urothelial carcinoma (UC). The ARON-2 study (NCT05290038) aimed to assess the real-world effectiveness of pembrolizumab in patients recurred or progressed after platinum-based chemotherapy.
Patients and methods: Medical records of patients with documented metastatic UC treated by pembrolizumab as second-line therapy were retrospectively collected from 88 institutions in 23 countries. Patients were assessed for overall survival (OS), progression-free survival (PFS) and overall response rate (ORR). Cox proportional hazards models were adopted to explore the presence of prognostic factors.
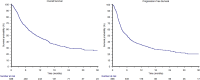
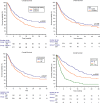
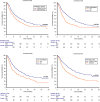
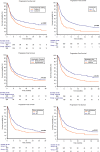
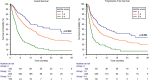
Results: In total, 836 patients were included: 544 patients (65%) received pembrolizumab after progression to first-line platinum-based chemotherapy in the metastatic setting (cohort A) and 292 (35%) after recurring within < 12 months since the completion of adjuvant or neoadjuvant chemotherapy (cohort B). The median follow-up time was 15.3 months. The median OS and the ORR were 10.5 months and 31% in the overall study population, 9.1 months and 29% in cohort A and 14.6 months and 37% in cohort B. At multivariate analysis, ECOG-PS ≥ 2, bone metastases, liver metastases and pembrolizumab setting (cohort A vs B) proved to be significantly associated with worst OS and PFS. Stratified by the presence of 0, 1-2 or 3-4 prognostic factors, the median OS was 29.4, 12.5 and 4.1 months (p < 0.001), while the median PFS was 12.2, 6.4 and 2.8 months, respectively (p < 0.001).
Conclusions: Our study confirms that pembrolizumab is effective in the advanced UC real-world context, showing outcome differences between patients recurred or progressed after platinum-based chemotherapy.
Keywords: ARON-2 study; Pembrolizumab; Real-world data; Survival; Tumor response; Urothelial cancer.
© 2024. The Author(s).
Conflict of interest statement
Matteo Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas and Bayer, all unrelated to the present paper. Francesco Massari has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD and Pfizer outside the submitted work. R. Kanesvaran has received fees for speaker bureau and advisory board activities from the following companies; Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas and Bayer. Enrique Grande has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Adacap, AMGEN, Angelini, Astellas, Astra Zeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, IPSEN, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho and Thermo Fisher Scientific. EG has received research grants from Pfizer, Astra Zeneca, Astellas and Lexicon Pharmaceuticals. Fernando Sabino Marques Monteiro has received research support from Janssen, Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb and Merck Sharp Dome. Camillo Porta has received honoraria from Angelini Pharma, AstraZeneca, BMS, Eisai, General Electric, Ipsen and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai and MSD. Sebastiano Buti received honoraria as speaker at scientific events and advisory role by BMS, Pfizer, MSD, Ipsen, AstraZeneca, Merck. Joaquim Bellmunt reports institutional and personal, paid and unpaid support for research, advisory boards, consultancy and honoraria (all outside this work) from: AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck, Pfizer, Roche, Sanofi/Aventis, Up-To-Date, CME events (Peerview, OncLive,), outside the submitted work. No speaker’s bureau. Javier Molina-Cerrillo declares consultant, advisory or speaker roles for IPSEN, Roche, Pfizer, Sanofi, Janssen and BMS. JMC has received research grants from Pfizer, IPSEN and Roche. Jose Carlos Tapia has received travel, accommodations, expenses from Roche, Pfizer, Merck and Lily. Zin Myint has received research support from Merck but not related to the present paper. Patrizia Giannatempo has received research support from Ipsen, Astra Zeneca e MSD and honoraria for speaker engagements, advisory roles from Astellas, MSD, Janssen, Pfizer. The other authors declare to have no conflicts of interest.
Figures
References
-
- https://gco.iarc.fr/ Accessed on October 8th, 2022
-
- Mohanty SK, Lobo A, Cheng L. The 2022 revision of World Health Organization classification of tumors of the urinary system and male genital organs: advances and challenges. Hum Pathol. 2022;S0046–8177(22):00224–226. - PubMed
-
- Powles T, Smith K, Stenzl A, Bedke J. Immune checkpoint inhibition in metastatic urothelial cancer. EurUrol. 2017;72:477–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

