SubSol-HIe is an AMPK-dependent hypoxia-responsive subnucleus of the nucleus tractus solitarius that coordinates the hypoxic ventilatory response and protects against apnoea in mice
- PMID: 38635058
- PMCID: PMC11166843
- DOI: 10.1007/s00424-024-02957-6
SubSol-HIe is an AMPK-dependent hypoxia-responsive subnucleus of the nucleus tractus solitarius that coordinates the hypoxic ventilatory response and protects against apnoea in mice
Abstract
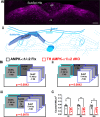
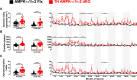
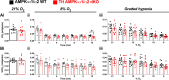
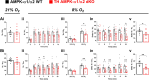
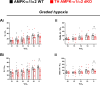
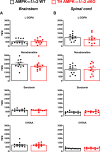
Functional magnetic resonance imaging (fMRI) suggests that the hypoxic ventilatory response is facilitated by the AMP-activated protein kinase (AMPK), not at the carotid bodies, but within a subnucleus (Bregma -7.5 to -7.1 mm) of the nucleus tractus solitarius that exhibits right-sided bilateral asymmetry. Here, we map this subnucleus using cFos expression as a surrogate for neuronal activation and mice in which the genes encoding the AMPK-α1 (Prkaa1) and AMPK-α2 (Prkaa2) catalytic subunits were deleted in catecholaminergic cells by Cre expression via the tyrosine hydroxylase promoter. Comparative analysis of brainstem sections, relative to controls, revealed that AMPK-α1/α2 deletion inhibited, with right-sided bilateral asymmetry, cFos expression in and thus activation of a neuronal cluster that partially spanned three interconnected anatomical nuclei adjacent to the area postrema: SolDL (Bregma -7.44 mm to -7.48 mm), SolDM (Bregma -7.44 mm to -7.48 mm) and SubP (Bregma -7.48 mm to -7.56 mm). This approximates the volume identified by fMRI. Moreover, these nuclei are known to be in receipt of carotid body afferent inputs, and catecholaminergic neurons of SubP and SolDL innervate aspects of the ventrolateral medulla responsible for respiratory rhythmogenesis. Accordingly, AMPK-α1/α2 deletion attenuated hypoxia-evoked increases in minute ventilation (normalised to metabolism), reductions in expiration time, and increases sigh frequency, but increased apnoea frequency during hypoxia. The metabolic response to hypoxia in AMPK-α1/α2 knockout mice and the brainstem and spinal cord catecholamine levels were equivalent to controls. We conclude that within the brainstem an AMPK-dependent, hypoxia-responsive subnucleus partially spans SubP, SolDM and SolDL, namely SubSol-HIe, and is critical to coordination of active expiration, the hypoxic ventilatory response and defence against apnoea.
Keywords: AMPK; Active expiration; Apnoea; Catecholaminergic; Hypoxia; Hypoxic ventilatory response; NTS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
AMPK facilitates the hypoxic ventilatory response through non-adrenergic mechanisms at the brainstem.Pflugers Arch. 2023 Jan;475(1):89-99. doi: 10.1007/s00424-022-02713-8. Epub 2022 Jun 10. Pflugers Arch. 2023. PMID: 35680670 Free PMC article.
-
AMP-activated Protein Kinase Deficiency Blocks the Hypoxic Ventilatory Response and Thus Precipitates Hypoventilation and Apnea.Am J Respir Crit Care Med. 2016 May 1;193(9):1032-43. doi: 10.1164/rccm.201508-1667OC. Am J Respir Crit Care Med. 2016. PMID: 26669206 Free PMC article.
-
O2-sensing after carotid chemodenervation: hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells.Eur J Neurosci. 2000 Sep;12(9):3181-90. doi: 10.1046/j.1460-9568.2000.00208.x. Eur J Neurosci. 2000. PMID: 10998102
-
The emerging role of AMPK in the regulation of breathing and oxygen supply.Biochem J. 2016 Sep 1;473(17):2561-72. doi: 10.1042/BCJ20160002. Biochem J. 2016. PMID: 27574022 Free PMC article. Review.
-
AMPK breathing and oxygen supply.Respir Physiol Neurobiol. 2019 Jul;265:112-120. doi: 10.1016/j.resp.2018.08.011. Epub 2018 Sep 19. Respir Physiol Neurobiol. 2019. PMID: 30243821 Review.
Cited by
-
Adaptive PRKAA1 variant in Andeans is associated with improved ventilation and sleep phenotypes.iScience. 2025 Jun 16;28(8):112911. doi: 10.1016/j.isci.2025.112911. eCollection 2025 Aug 15. iScience. 2025. PMID: 40703441 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

