An Active Site Tyr Residue Guides the Regioselectivity of Lysine Hydroxylation by Nonheme Iron Lysine-4-hydroxylase Enzymes through Proton-Coupled Electron Transfer
- PMID: 38636166
- PMCID: PMC11066847
- DOI: 10.1021/jacs.3c14574
An Active Site Tyr Residue Guides the Regioselectivity of Lysine Hydroxylation by Nonheme Iron Lysine-4-hydroxylase Enzymes through Proton-Coupled Electron Transfer
Abstract
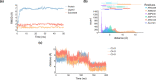
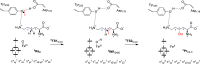
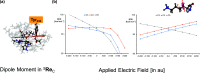
Lysine dioxygenase (KDO) is an important enzyme in human physiology involved in bioprocesses that trigger collagen cross-linking and blood pressure control. There are several KDOs in nature; however, little is known about the factors that govern the regio- and stereoselectivity of these enzymes. To understand how KDOs can selectively hydroxylate their substrate, we did a comprehensive computational study into the mechanisms and features of 4-lysine dioxygenase. In particular, we selected a snapshot from the MD simulation on KDO5 and created large QM cluster models (A, B, and C) containing 297, 312, and 407 atoms, respectively. The largest model predicts regioselectivity that matches experimental observation with rate-determining hydrogen atom abstraction from the C4-H position, followed by fast OH rebound to form 4-hydroxylysine products. The calculations show that in model C, the dipole moment is positioned along the C4-H bond of the substrate and, therefore, the electrostatic and electric field perturbations of the protein assist the enzyme in creating C4-H hydroxylation selectivity. Furthermore, an active site Tyr233 residue is identified that reacts through proton-coupled electron transfer akin to the axial Trp residue in cytochrome c peroxidase. Thus, upon formation of the iron(IV)-oxo species in the catalytic cycle, the Tyr233 phenol loses a proton to the nearby Asp179 residue, while at the same time, an electron is transferred to the iron to create an iron(III)-oxo active species. This charged tyrosyl residue directs the dipole moment along the C4-H bond of the substrate and guides the selectivity to the C4-hydroxylation of the substrate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Local Charge Distributions, Electric Dipole Moments, and Local Electric Fields Influence Reactivity Patterns and Guide Regioselectivities in α-Ketoglutarate-Dependent Non-heme Iron Dioxygenases.Acc Chem Res. 2022 Jan 4;55(1):65-74. doi: 10.1021/acs.accounts.1c00538. Epub 2021 Dec 17. Acc Chem Res. 2022. PMID: 34915695 Review.
-
What is the Origin of the Regioselective C3-Hydroxylation of L-Arg by the Nonheme Iron Enzyme Capreomycin C?Chemistry. 2024 Nov 26;30(66):e202402604. doi: 10.1002/chem.202402604. Epub 2024 Oct 16. Chemistry. 2024. PMID: 39190221
-
How Do Electrostatic Perturbations of the Protein Affect the Bifurcation Pathways of Substrate Hydroxylation versus Desaturation in the Nonheme Iron-Dependent Viomycin Biosynthesis Enzyme?J Phys Chem A. 2021 Mar 4;125(8):1720-1737. doi: 10.1021/acs.jpca.1c00141. Epub 2021 Feb 23. J Phys Chem A. 2021. PMID: 33620220
-
Origin of the correlation of the rate constant of substrate hydroxylation by nonheme iron(IV)-oxo complexes with the bond-dissociation energy of the C-H bond of the substrate.Chemistry. 2009 Jul 6;15(27):6651-62. doi: 10.1002/chem.200900211. Chemistry. 2009. PMID: 19472231
-
Second-Coordination Sphere Effects on Selectivity and Specificity of Heme and Nonheme Iron Enzymes.Chemistry. 2020 Apr 24;26(24):5308-5327. doi: 10.1002/chem.201905119. Epub 2020 Feb 19. Chemistry. 2020. PMID: 31804749 Review.
Cited by
-
Nitrile Hydroboration by Cooperative Iron Catalysis: An Experimental and Computational Study.Chemistry. 2025 Jul 22;31(41):e202501782. doi: 10.1002/chem.202501782. Epub 2025 Jul 2. Chemistry. 2025. PMID: 40553511 Free PMC article.
-
Origins of Catalysis in Non-Heme Fe(II)/2-Oxoglutarate-Dependent Histone Lysine Demethylase KDM4A with Differently Methylated Histone H3 Peptides.Chemistry. 2025 Jan 14;31(3):e202403989. doi: 10.1002/chem.202403989. Epub 2024 Nov 18. Chemistry. 2025. PMID: 39487094 Free PMC article.
-
Mechanism of the Oxidative Ring-Closure Reaction during Gliotoxin Biosynthesis by Cytochrome P450 GliF.Int J Mol Sci. 2024 Aug 6;25(16):8567. doi: 10.3390/ijms25168567. Int J Mol Sci. 2024. PMID: 39201254 Free PMC article.
-
Probing Ferryl Reactivity in a Nonheme Iron Oxygenase Using an Expanded Genetic Code.ACS Catal. 2024 Jul 20;14(15):11584-11590. doi: 10.1021/acscatal.4c02365. eCollection 2024 Aug 2. ACS Catal. 2024. PMID: 39114090 Free PMC article.
-
easyPARM: Automated, Versatile, and Reliable Force Field Parameters for Metal-Containing Molecules with Unique Labeling of Coordinating Atoms.J Chem Theory Comput. 2025 Feb 25;21(4):1817-1830. doi: 10.1021/acs.jctc.4c01272. Epub 2025 Feb 6. J Chem Theory Comput. 2025. PMID: 39913238 Free PMC article.
References
-
- Kasamatsu A.; Uzawa K.; Hayashi F.; Kita A.; Okubo Y.; Saito T.; Kimura Y.; Miyamoto I.; Oka N.; Shiiba M.; Ito C.; Toshimori K.; Miki T.; Yamauchi M.; Tanzawa H. Deficiency of Lysyl Hydroxylase 2 in Mice Causes Systemic Endoplasmic Reticulum Stress Leading to Early Embryonic Lethality. Biochem. Biophys. Res. Commun. 2019, 512, 486–491. 10.1016/j.bbrc.2019.03.091. - DOI - PubMed
-
- Gong S.; Wu C.; Köhler F.; Meixensberger J.; Schopow N.; Kallendrusch S. Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase Family: Novel Prognostic Biomarkers and Tumor Microenvironment Regulators for Lower-Grade Glioma. Front. Cell. Neurosci. 2022, 16, 83854810.3389/fncel.2022.838548. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

