Genomic Analysis and Surveillance of Respiratory Syncytial Virus Using Wastewater-Based Epidemiology
- PMID: 38636496
- PMCID: PMC11481326
- DOI: 10.1093/infdis/jiae205
Genomic Analysis and Surveillance of Respiratory Syncytial Virus Using Wastewater-Based Epidemiology
Abstract
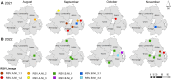
Respiratory syncytial virus (RSV) causes severe infections in infants, immunocompromised or elderly individuals resulting in annual epidemics of respiratory disease. Currently, limited clinical surveillance and the lack of predictable seasonal dynamics limit the public health response. Wastewater-based epidemiology (WBE) has recently been used globally as a key metric in determining prevalence of severe acute respiratory syndrome coronavirus 2 in the community, but its application to other respiratory viruses is limited. In this study, we present an integrated genomic WBE approach, applying reverse-transcription quantitative polymerase chain reaction and partial G-gene sequencing to track RSV levels and variants in the community. We report increasing detection of RSV in wastewater concomitant with increasing numbers of positive clinical cases. Analysis of wastewater-derived RSV sequences permitted identification of distinct circulating lineages within and between seasons. Altogether, our genomic WBE platform has the potential to complement ongoing global surveillance and aid the management of RSV by informing the timely deployment of pharmaceutical and nonpharmaceutical interventions.
Keywords: RSV; genome; respiratory syncytial virus; surveillance; wastewater.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H, Investigators R. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis 2022; 226(suppl 1):S17–21. - PubMed
-
- Hammitt LL, Dagan R, Yuan Y, et al. . Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386:837–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

