Pseudorabies virus manipulates mitochondrial tryptophanyl-tRNA synthetase 2 for viral replication
- PMID: 38636706
- PMCID: PMC11279775
- DOI: 10.1016/j.virs.2024.04.003
Pseudorabies virus manipulates mitochondrial tryptophanyl-tRNA synthetase 2 for viral replication
Abstract
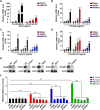
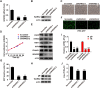
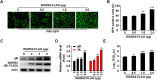
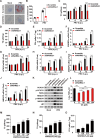
The pseudorabies virus (PRV) is identified as a double-helical DNA virus responsible for causing Aujeszky's disease, which results in considerable economic impacts globally. The enzyme tryptophanyl-tRNA synthetase 2 (WARS2), a mitochondrial protein involved in protein synthesis, is recognized for its broad expression and vital role in the translation process. The findings of our study showed an increase in both mRNA and protein levels of WARS2 following PRV infection in both cell cultures and animal models. Suppressing WARS2 expression via RNA interference in PK-15 cells led to a reduction in PRV infection rates, whereas enhancing WARS2 expression resulted in increased infection rates. Furthermore, the activation of WARS2 in response to PRV was found to be reliant on the cGAS/STING/TBK1/IRF3 signaling pathway and the interferon-alpha receptor-1, highlighting its regulation via the type I interferon signaling pathway. Further analysis revealed that reducing WARS2 levels hindered PRV's ability to promote protein and lipid synthesis. Our research provides novel evidence that WARS2 facilitates PRV infection through its management of protein and lipid levels, presenting new avenues for developing preventative and therapeutic measures against PRV infections.
Keywords: Innate immunity; Lipid synthesis; Protein synthesis; Pseudorabies virus; WARS2.
Copyright © 2024 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no competing interests.
Figures



Similar articles
-
Inhibition of PARP1 Dampens Pseudorabies Virus Infection through DNA Damage-Induced Antiviral Innate Immunity.J Virol. 2021 Jul 26;95(16):e0076021. doi: 10.1128/JVI.00760-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34037418 Free PMC article.
-
Porcine IFITM1 is a host restriction factor that inhibits pseudorabies virus infection.Int J Biol Macromol. 2020 May 15;151:1181-1193. doi: 10.1016/j.ijbiomac.2019.10.162. Epub 2019 Nov 16. Int J Biol Macromol. 2020. PMID: 31743714 Free PMC article.
-
Pseudorabies virus infection triggers mitophagy to dampen the interferon response and promote viral replication.J Virol. 2024 Oct 22;98(10):e0104824. doi: 10.1128/jvi.01048-24. Epub 2024 Aug 30. J Virol. 2024. PMID: 39212384 Free PMC article.
-
Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine.Microbiol Mol Biol Rev. 2005 Sep;69(3):462-500. doi: 10.1128/MMBR.69.3.462-500.2005. Microbiol Mol Biol Rev. 2005. PMID: 16148307 Free PMC article. Review.
-
A Tug of War: Pseudorabies Virus and Host Antiviral Innate Immunity.Viruses. 2022 Mar 6;14(3):547. doi: 10.3390/v14030547. Viruses. 2022. PMID: 35336954 Free PMC article. Review.
Cited by
-
Insight into the Interaction Mechanism of Pseudorabies Virus Infection.Biology (Basel). 2024 Dec 4;13(12):1013. doi: 10.3390/biology13121013. Biology (Basel). 2024. PMID: 39765680 Free PMC article. Review.
-
Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus.Int J Mol Sci. 2025 Mar 23;26(7):2912. doi: 10.3390/ijms26072912. Int J Mol Sci. 2025. PMID: 40243525 Free PMC article.
References
-
- Antonellis A., Green E. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu. Rev. Genomics Hum. Genet. 2008;9:87–107. - PubMed
-
- Bonnevie-Nielsen V., Gerdes A., Fleckner J., Petersen J., Michelsen B., Dyrberg T. Interferon stimulates the expression of 2',5'-oligoadenylate synthetase and MHC class I antigens in insulin-producing cells. J. Interferon Res. 1991;11:255–260. - PubMed
-
- Burke E., Frucht S., Thompson K., Wolfe L., Yokoyama T., Bertoni M., Huang Y., Sincan M., Adams D., Taylor R., Gahl W., Toro C., Malicdan M. Biallelic mutations in mitochondrial tryptophanyl-tRNA synthetase cause Levodopa-responsive infantile-onset Parkinsonism. Clin. Genet. 2018;93:712–718. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

