Circuit-Wide Gene Network Analysis Reveals Sex-Specific Roles for Phosphodiesterase 1b in Cocaine Addiction
- PMID: 38637154
- PMCID: PMC11154853
- DOI: 10.1523/JNEUROSCI.1327-23.2024
Circuit-Wide Gene Network Analysis Reveals Sex-Specific Roles for Phosphodiesterase 1b in Cocaine Addiction
Abstract
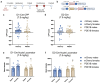
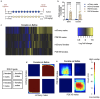
Cocaine use disorder is a significant public health issue without an effective pharmacological treatment. Successful treatments are hindered in part by an incomplete understanding of the molecular mechanisms that underlie long-lasting maladaptive plasticity and addiction-like behaviors. Here, we leverage a large RNA sequencing dataset to generate gene coexpression networks across six interconnected regions of the brain's reward circuitry from mice that underwent saline or cocaine self-administration. We identify phosphodiesterase 1b (Pde1b), a Ca2+/calmodulin-dependent enzyme that increases cAMP and cGMP hydrolysis, as a central hub gene within a nucleus accumbens (NAc) gene module that was bioinformatically associated with addiction-like behavior. Chronic cocaine exposure increases Pde1b expression in NAc D2 medium spiny neurons (MSNs) in male but not female mice. Viral-mediated Pde1b overexpression in NAc reduces cocaine self-administration in female rats but increases seeking in both sexes. In female mice, overexpressing Pde1b in D1 MSNs attenuates the locomotor response to cocaine, with the opposite effect in D2 MSNs. Overexpressing Pde1b in D1/D2 MSNs had no effect on the locomotor response to cocaine in male mice. At the electrophysiological level, Pde1b overexpression reduces sEPSC frequency in D1 MSNs and regulates the excitability of NAc MSNs. Lastly, Pde1b overexpression significantly reduced the number of differentially expressed genes (DEGs) in NAc following chronic cocaine, with discordant effects on gene transcription between sexes. Together, we identify novel gene modules across the brain's reward circuitry associated with addiction-like behavior and explore the role of Pde1b in regulating the molecular, cellular, and behavioral responses to cocaine.
Keywords: addiction; bioinformatics; cocaine; genes; phosphodiesterase; plasticity.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
