The incidences of nausea and vomiting after general anesthesia with remimazolam versus sevoflurane: a prospective randomized controlled trial
- PMID: 38637272
- PMCID: PMC11294881
- DOI: 10.4097/kja.23939
The incidences of nausea and vomiting after general anesthesia with remimazolam versus sevoflurane: a prospective randomized controlled trial
Abstract
Background: Postoperative nausea and vomiting (PONV) refers to nausea and vomiting that occurs within 24-h after surgery or in the post-anesthesia care unit (PACU). Previous studies have reported that the use of remimazolam, a newer benzodiazepine (BDZ) hypnotic, for anesthesia results in less PONV. In this study, we compared the rate of PONV between sevoflurane and remimazolam after general anesthesia.
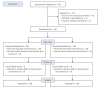
Methods: In this prospective randomized controlled trial, participants aged 20-80 years who underwent elective laparoscopic cholecystectomy or hemicolectomy were randomized to either the remimazolam or sevoflurane group. The primary outcome was PONV incidence for 24-h after surgery. Secondary outcomes comprised of PONV at 30-min post-surgery, postoperative additional antiemetic use, and Quality of Recovery-15 (QOR-15) score at 24-h postoperatively.
Results: Forty patients were enrolled in the study. The remimazolam group exhibited significantly lower rates of PONV for 24-h after surgery than did the sevoflurane group (remimazolam group vs. sevoflurane group; 5% vs. 45%, P = 0.003, respectively). The use of dexamethasone, a rescue antiemetic administered within 24 h of surgery, was substantially lower in the remimazolam group than in the sevoflurane group (0% in remimazolam vs. 30% in sevoflurane, P = 0.020). The QOR-15 score at 24-h after surgery showed no significant difference between the two groups.
Conclusions: Compared to sevoflurane, opting for remimazolam as an intraoperative hypnotic may decrease the incidence of PONV and reduce antiemetic use for 24 h after laparoscopic surgery.
Keywords: Analgesics; Antiemetics; Benzodiazepines; Nausea; Sevoflurane; Vomiting.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132:636–51. - PubMed
-
- Ahn EJ, Kang H, Choi GJ, Baek CW, Jung YH, Woo YC. The effectiveness of midazolam for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Anesth Analg. 2016;122:664–76. - PubMed
-
- Greene NH, Habib AS. Midazolam for anxiolysis and postoperative nausea and vomiting prophylaxis: can we kill two birds with one stone? Anesth Analg. 2016;122:590–2. - PubMed
-
- Heidari SM, Saryazdi H, Saghaei M. Effect of intravenous midazolam premedication on postoperative nausea and vomiting after cholecystectomy. Acta Anaesthesiol Taiwan. 2004;42:77–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources