Neutrophil Extracellular Traps Regulate Surgical Brain Injury by Activating the cGAS-STING Pathway
- PMID: 38637346
- PMCID: PMC11026279
- DOI: 10.1007/s10571-024-01470-9
Neutrophil Extracellular Traps Regulate Surgical Brain Injury by Activating the cGAS-STING Pathway
Abstract
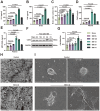
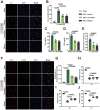
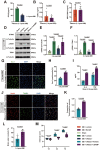
Surgical brain injury (SBI), induced by neurosurgical procedures or instruments, has not attracted adequate attention. The pathophysiological process of SBI remains sparse compared to that of other central nervous system diseases thus far. Therefore, novel and effective therapies for SBI are urgently needed. In this study, we found that neutrophil extracellular traps (NETs) were present in the circulation and brain tissues of rats after SBI, which promoted neuroinflammation, cerebral edema, neuronal cell death, and aggravated neurological dysfunction. Inhibition of NETs formation by peptidylarginine deiminase (PAD) inhibitor or disruption of NETs with deoxyribonuclease I (DNase I) attenuated SBI-induced damages and improved the recovery of neurological function. We show that SBI triggered the activation of cyclic guanosine monophosphate-adenosine monophosphate synthase stimulator of interferon genes (cGAS-STING), and that inhibition of the cGAS-STING pathway could be beneficial. It is worth noting that DNase I markedly suppressed the activation of cGAS-STING, which was reversed by the cGAS product cyclic guanosine monophosphate-adenosine monophosphate (cGMP-AMP, cGAMP). Furthermore, the neuroprotective effect of DNase I in SBI was also abolished by cGAMP. NETs may participate in the pathophysiological regulation of SBI by acting through the cGAS-STING pathway. We also found that high-dose vitamin C administration could effectively inhibit the formation of NETs post-SBI. Thus, targeting NETs may provide a novel therapeutic strategy for SBI treatment, and high-dose vitamin C intervention may be a promising translational therapy with an excellent safety profile and low cost.
Keywords: Neutrophil extracellular traps; STING; Surgical brain injury; Vitamin C; cGAS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Cyclic guanosine monophosphate-adenosine monophosphate synthetase/stimulator of interferon genes signaling aggravated corneal allograft rejection through neutrophil extracellular traps.Am J Transplant. 2024 Sep;24(9):1583-1596. doi: 10.1016/j.ajt.2024.04.010. Epub 2024 Apr 20. Am J Transplant. 2024. PMID: 38648890
-
Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury.J Neuroinflammation. 2023 Sep 30;20(1):222. doi: 10.1186/s12974-023-02903-w. J Neuroinflammation. 2023. PMID: 37777772 Free PMC article.
-
Smart Liposomal Nanocarrier Enhanced the Treatment of Ischemic Stroke through Neutrophil Extracellular Traps and Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Genes (cGAS-STING) Pathway Inhibition of Ischemic Penumbra.ACS Nano. 2023 Sep 26;17(18):17845-17857. doi: 10.1021/acsnano.3c03390. Epub 2023 Sep 15. ACS Nano. 2023. PMID: 37712845
-
The role of cGAS-STING signalling in liver diseases.JHEP Rep. 2021 Jun 24;3(5):100324. doi: 10.1016/j.jhepr.2021.100324. eCollection 2021 Oct. JHEP Rep. 2021. PMID: 34381984 Free PMC article. Review.
-
Cell-to-cell communications of cGAS-STING pathway in tumor immune microenvironment.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 12;53(1):15-24. doi: 10.3724/zdxbyxb-2023-0482. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38229499 Free PMC article. Review. Chinese, English.
Cited by
-
Transcriptomic profile of microglia following inflammation-sensitized hypoxic-ischemic brain injury in neonatal rats suggests strong contribution to neutrophil chemotaxis and activation.J Neuroinflammation. 2025 Jul 19;22(1):189. doi: 10.1186/s12974-025-03516-1. J Neuroinflammation. 2025. PMID: 40684206 Free PMC article.
-
Irisin regulates integrin αvβ5/FAK/ERK to inhibit neutrophil extracellular traps formation and reduce pancreatic beta-cells pyroptosis in type 2 diabetes mellitus.Diabetol Metab Syndr. 2025 Jul 18;17(1):279. doi: 10.1186/s13098-025-01852-z. Diabetol Metab Syndr. 2025. PMID: 40682113 Free PMC article.
-
Lactate-induced macrophage HMGB1 lactylation promotes neutrophil extracellular trap formation in sepsis-associated acute kidney injury.Cell Biol Toxicol. 2025 Apr 30;41(1):78. doi: 10.1007/s10565-025-10026-6. Cell Biol Toxicol. 2025. PMID: 40304798 Free PMC article.
-
Investigating the Role of Neutrophil Extracellular Traps as a Therapeutic Target in Traumatic Brain Injury: a Systematic Review and Meta-analysis.Mol Neurobiol. 2025 May 23. doi: 10.1007/s12035-025-05053-7. Online ahead of print. Mol Neurobiol. 2025. PMID: 40408025 Review.
References
-
- Apel F, Andreeva L, Knackstedt LS, Streeck R, Frese CK, Goosmann C, Hopfner KP, Zychlinsky A (2021) The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci Signal. 10.1126/scisignal.aax7942 - PubMed
-
- Bahrami Z, Firouzi M, Hashemi-Monfared A, Zahednasab H, Harirchian MH (2018) The effect of minocycline on indolamine 2, 3 dioxygenase expression and the levels of kynurenic acid and quinolinic acid in LPS-activated primary rat microglia. Cytokine 107:125–129. 10.1016/j.cyto.2017.12.013 - PubMed
-
- Chang CY, Chen JY, Wu MH, Hu ML (2020) Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic Biol Med 155:29–36. 10.1016/j.freeradbiomed.2020.05.015 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

