Local environment in biomolecular condensates modulates enzymatic activity across length scales
- PMID: 38637545
- PMCID: PMC11026464
- DOI: 10.1038/s41467-024-47435-w
Local environment in biomolecular condensates modulates enzymatic activity across length scales
Abstract
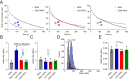
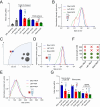
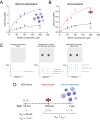
The mechanisms that underlie the regulation of enzymatic reactions by biomolecular condensates and how they scale with compartment size remain poorly understood. Here we use intrinsically disordered domains as building blocks to generate programmable enzymatic condensates of NADH-oxidase (NOX) with different sizes spanning from nanometers to microns. These disordered domains, derived from three distinct RNA-binding proteins, each possessing different net charge, result in the formation of condensates characterized by a comparable high local concentration of the enzyme yet within distinct environments. We show that only condensates with the highest recruitment of substrate and cofactor exhibit an increase in enzymatic activity. Notably, we observe an enhancement in enzymatic rate across a wide range of condensate sizes, from nanometers to microns, indicating that emergent properties of condensates can arise within assemblies as small as nanometers. Furthermore, we show a larger rate enhancement in smaller condensates. Our findings demonstrate the ability of condensates to modulate enzymatic reactions by creating distinct effective solvent environments compared to the surrounding solution, with implications for the design of protein-based heterogeneous biocatalysts.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures