ADNP dysregulates methylation and mitochondrial gene expression in the cerebellum of a Helsmoortel-Van der Aa syndrome autopsy case
- PMID: 38637827
- PMCID: PMC11027339
- DOI: 10.1186/s40478-024-01743-w
ADNP dysregulates methylation and mitochondrial gene expression in the cerebellum of a Helsmoortel-Van der Aa syndrome autopsy case
Erratum in
-
Correction to: ADNP dysregulates methylation and mitochondrial gene expression in the cerebellum of a Helsmoortel-Van Der Aa syndrome autopsy case.Acta Neuropathol Commun. 2024 Oct 24;12(1):168. doi: 10.1186/s40478-024-01787-y. Acta Neuropathol Commun. 2024. PMID: 39449121 Free PMC article. No abstract available.
Abstract
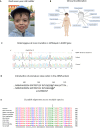
Background: Helsmoortel-Van der Aa syndrome is a neurodevelopmental disorder in which patients present with autism, intellectual disability, and frequent extra-neurological features such as feeding and gastrointestinal problems, visual impairments, and cardiac abnormalities. All patients exhibit heterozygous de novo nonsense or frameshift stop mutations in the Activity-Dependent Neuroprotective Protein (ADNP) gene, accounting for a prevalence of 0.2% of all autism cases worldwide. ADNP fulfills an essential chromatin remodeling function during brain development. In this study, we investigated the cerebellum of a died 6-year-old male patient with the c.1676dupA/p.His559Glnfs*3 ADNP mutation.
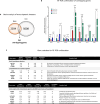
Results: The clinical presentation of the patient was representative of the Helsmoortel-Van der Aa syndrome. During his lifespan, he underwent two liver transplantations after which the child died because of multiple organ failure. An autopsy was performed, and various tissue samples were taken for further analysis. We performed a molecular characterization of the cerebellum, a brain region involved in motor coordination, known for its highest ADNP expression and compared it to an age-matched control subject. Importantly, epigenome-wide analysis of the ADNP cerebellum identified CpG methylation differences and expression of multiple pathways causing neurodevelopmental delay. Interestingly, transcription factor motif enrichment analysis of differentially methylated genes showed that the ADNP binding motif was the most significantly enriched. RNA sequencing of the autopsy brain further identified downregulation of the WNT signaling pathway and autophagy defects as possible causes of neurodevelopmental delay. Ultimately, label-free quantification mass spectrometry identified differentially expressed proteins involved in mitochondrial stress and sirtuin signaling pathways amongst others. Protein-protein interaction analysis further revealed a network including chromatin remodelers (ADNP, SMARCC2, HDAC2 and YY1), autophagy-related proteins (LAMP1, BECN1 and LC3) as well as a key histone deacetylating enzyme SIRT1, involved in mitochondrial energy metabolism. The protein interaction of ADNP with SIRT1 was further biochemically validated through the microtubule-end binding proteins EB1/EB3 by direct co-immunoprecipitation in mouse cerebellum, suggesting important mito-epigenetic crosstalk between chromatin remodeling and mitochondrial energy metabolism linked to autophagy stress responses. This is further supported by mitochondrial activity assays and stainings in patient-derived fibroblasts which suggest mitochondrial dysfunctions in the ADNP deficient human brain.
Conclusion: This study forms the baseline clinical and molecular characterization of an ADNP autopsy cerebellum, providing novel insights in the disease mechanisms of the Helsmoortel-Van der Aa syndrome. By combining multi-omic and biochemical approaches, we identified a novel SIRT1-EB1/EB3-ADNP protein complex which may contribute to autophagic flux alterations and impaired mitochondrial metabolism in the Helsmoortel-Van der Aa syndrome and holds promise as a new therapeutic target.
Keywords: Activity-dependent neuroprotective protein (ADNP); Autophagy; Chromatin remodeler; Helsmoortel–Van der Aa syndrome; Methylation; Mitochondria; Post-mortem brain; Sirtuin 1 (SIRT1).
© 2024. The Author(s).
Conflict of interest statement
Professor Dr. Em. Illana Gozes serves as VP drug development at ExoNavis Therapeutics LTD, clinically developing Davunetide.
Figures








References
-
- Vissers LELM, de Ligt J, Gilissen C et al (2010) A de novo paradigm for mental retardation. Nat Genet 42:1109–1112. 10.1038/ng.712 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

