Discovery of a novel and highly selective JAK3 inhibitor as a potent hair growth promoter
- PMID: 38637842
- PMCID: PMC11025159
- DOI: 10.1186/s12967-024-05144-4
Discovery of a novel and highly selective JAK3 inhibitor as a potent hair growth promoter
Abstract
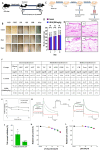
JAK-STAT signalling pathway inhibitors have emerged as promising therapeutic agents for the treatment of hair loss. Among different JAK isoforms, JAK3 has become an ideal target for drug discovery because it only regulates a narrow spectrum of γc cytokines. Here, we report the discovery of MJ04, a novel and highly selective 3-pyrimidinylazaindole based JAK3 inhibitor, as a potential hair growth promoter with an IC50 of 2.03 nM. During in vivo efficacy assays, topical application of MJ04 on DHT-challenged AGA and athymic nude mice resulted in early onset of hair regrowth. Furthermore, MJ04 significantly promoted the growth of human hair follicles under ex-vivo conditions. MJ04 exhibited a reasonably good pharmacokinetic profile and demonstrated a favourable safety profile under in vivo and in vitro conditions. Taken together, we report MJ04 as a highly potent and selective JAK3 inhibitor that exhibits overall properties suitable for topical drug development and advancement to human clinical trials.
Keywords: 3-pyrimidinylazaindole; Alopecia areata; Baricitinib; Hair follicles; JAK-STAT signalling; Ritlecitinib; Tofacitinib.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
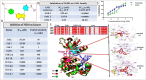


 with very tiny hair follicles
with very tiny hair follicles  . c. Minimal epidermal thinning with few hair follicles on Days 7 and 14 and increased hair follicles on Days 21 and 28
. c. Minimal epidermal thinning with few hair follicles on Days 7 and 14 and increased hair follicles on Days 21 and 28  . d. Minimal degree of epidermal thickening with increased distinct hair follicles
. d. Minimal degree of epidermal thickening with increased distinct hair follicles  in the dermal and hypodermal regions. e. Minimal degree of epidermal atrophy
in the dermal and hypodermal regions. e. Minimal degree of epidermal atrophy  with hair follicles on Days 7 and 14 and minimal epidermal thickening
with hair follicles on Days 7 and 14 and minimal epidermal thickening  with increased hair follicles
with increased hair follicles  on Days 21 and 28. f. Normal epidermis with increased hair follicles
on Days 21 and 28. f. Normal epidermis with increased hair follicles  on Days 14 and 28. D Mean Weekly Body Weight: Monitoring of mean weekly body weight of DHT induced AGA mice model throughout the evaluation of MJ04’s impact on hair regrowth. Here, the groups are denoted as follows a = Control; b = Testosterone treated; c = Tofacitinib treated (0.08mg/kg), d = Baricitinib treated (0.04mg/kg), e = MJ04 treated (0.04mg/kg) and f = MJ04 treated (0.08mg/kg). The body weight of the induced AGA mice model was recorded on days 1, 7, 14, 21, and 28
on Days 14 and 28. D Mean Weekly Body Weight: Monitoring of mean weekly body weight of DHT induced AGA mice model throughout the evaluation of MJ04’s impact on hair regrowth. Here, the groups are denoted as follows a = Control; b = Testosterone treated; c = Tofacitinib treated (0.08mg/kg), d = Baricitinib treated (0.04mg/kg), e = MJ04 treated (0.04mg/kg) and f = MJ04 treated (0.08mg/kg). The body weight of the induced AGA mice model was recorded on days 1, 7, 14, 21, and 28
 , dermis
, dermis  , hypodermis
, hypodermis  , panniculus carnosus, and adventitia
, panniculus carnosus, and adventitia  . c and d (Tofacitinib citrate—0.08 and 0.8 mg/kg): Mild epidermal thickening with the increased number of hair follicles [
. c and d (Tofacitinib citrate—0.08 and 0.8 mg/kg): Mild epidermal thickening with the increased number of hair follicles [ ] (++++). e (MJ04—0.08 mg/kg): Minimal epidermal thickening with moderate hair follicle (++). f (MJ04—0.04 mg/kg): Mild epidermal thinning with increased hair follicles in the dermis and hypodermis (++++). g (MJ04—0.016 mg/kg): Mild epidermal thickening with increased hair follicles (+++). h (Baricitinib–0.1 mg/kg): Increased and morphologically distinct hair follicles
] (++++). e (MJ04—0.08 mg/kg): Minimal epidermal thickening with moderate hair follicle (++). f (MJ04—0.04 mg/kg): Mild epidermal thinning with increased hair follicles in the dermis and hypodermis (++++). g (MJ04—0.016 mg/kg): Mild epidermal thickening with increased hair follicles (+++). h (Baricitinib–0.1 mg/kg): Increased and morphologically distinct hair follicles  in the dermis and hypodermis regions (+++). i and j (Baricitinib—0.04 and 0.02 mg/kg): Moderate degree of epidermal thickening
in the dermis and hypodermis regions (+++). i and j (Baricitinib—0.04 and 0.02 mg/kg): Moderate degree of epidermal thickening  with more hair follicles in the dermis and hypodermis regions (++++), and D MJ04 promotes the growth of human hair follicles under ex-vivo conditions. (a) Control group: (i) Basal length, (ii) Length posttreatment, (b) Experimental group treated with 100 μl of MJ04 (0.50 μg/μl): (i) Basal length, (ii) Length post-experiment, and (c) Experimental group treated with 100 μl of MJ04 (0.25 μg/μl): (i) Basal length, (ii) Length post-treatment. The red bracket indicates the length of the human hair follicle
with more hair follicles in the dermis and hypodermis regions (++++), and D MJ04 promotes the growth of human hair follicles under ex-vivo conditions. (a) Control group: (i) Basal length, (ii) Length posttreatment, (b) Experimental group treated with 100 μl of MJ04 (0.50 μg/μl): (i) Basal length, (ii) Length post-experiment, and (c) Experimental group treated with 100 μl of MJ04 (0.25 μg/μl): (i) Basal length, (ii) Length post-treatment. The red bracket indicates the length of the human hair follicle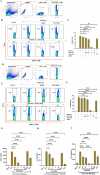
References
-
- Sadick NS, Callender VD, Kircik LH, Kogan SJ. New insight into the pathophysiology of hair loss trigger a paradigm shift in the treatment approach. J Drugs Dermatol. 2017;16:s135–s140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

