Treatment of COVID-19-associated ARDS with umbilical cord-derived mesenchymal stromal cells in the STROMA-CoV-2 multicenter randomized double-blind trial: long-term safety, respiratory function, and quality of life
- PMID: 38637891
- PMCID: PMC11027516
- DOI: 10.1186/s13287-024-03729-w
Treatment of COVID-19-associated ARDS with umbilical cord-derived mesenchymal stromal cells in the STROMA-CoV-2 multicenter randomized double-blind trial: long-term safety, respiratory function, and quality of life
Abstract
Background: The STROMA-CoV-2 study was a French phase 2b, multicenter, double-blind, randomized, placebo-controlled clinical trial that did not identify a significant efficacy of umbilical cord-derived mesenchymal stromal cells in patients with SARS-CoV-2-induced acute respiratory distress syndrome. Safety on day 28 was found to be good. The aim of our extended study was to assess the 6- and 12-month safety of UC-MSCs administration in the STROMA-CoV-2 cohort.
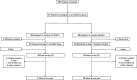
Methods: A detailed multi-domain assessment was conducted at 6 and 12 months following hospital discharge focusing on adverse events, lung computed tomography-scan, pulmonary and muscular functional status, and quality of life in the STROMA-CoV-2 cohort including SARS-CoV-2-related early (< 96 h) mild-to-severe acute respiratory distress syndrome.
Results: Between April 2020 and October 2020, 47 patients were enrolled, of whom 19 completed a 1-year follow-up. There were no significant differences in any endpoints or adverse effects between the UC-MSCs and placebo groups at the 6- and 12-month assessments. Ground-glass opacities persisted at 1 year in 5 patients (26.3%). Furthermore, diffusing capacity for carbon monoxide remained altered over 1 year, although no patient required oxygen or non-invasive ventilatory support. Quality of life revealed declines in mental, emotional and physical health throughout the follow-up period, and the six-minute walking distance remained slightly impaired at the 1-year patient assessment.
Conclusions: This study suggests a favorable safety profile for the use of intravenous UC-MSCs in the context of the first French wave of SARS-CoV-2-related moderate-to-severe acute respiratory distress syndrome, with no adverse effects observed at 1 year.
Keywords: Acute respiratory distress syndrome; Follow-up Studies; Long-term outcomes; Quality of Life at six and twelve months after hospital discharge; Severe acute respiratory syndrome coronavirus‐2; Umbilical cord‐ derived mesenchymal stromal cells.
© 2024. The Author(s).
Conflict of interest statement
AD declared grants or contracts from Philips, Fisher & Paykel; French Ministry of Health; Respinor; Lungspacer; consulting fees from Lungspacer, Respinor; payments or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Fisher & Paykel, Getinge, Lungspacer, Gilead, Lowenstein, Astra; support for attending meetings and/or travel from Fisher & Paykel, Lungspacer; received equipment, materials, drugs, medical writing, gifts or other services from Lungspacer, Respinor. MF declared grants or contracts from BioMérieux and MSD; French Ministry of Health; consulting fees from Pfizer; payments or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Fisher & Paykel and Biomérieux; participation on a data safety monitoring board or advisory board. No conflict of interests is reported for other authors.
Figures

References
-
- Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care [Internet]. 2022;26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8860258/. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

