Small fibre neuropathy in Fabry disease: a human-derived neuronal in vitro disease model and pilot data
- PMID: 38638148
- PMCID: PMC11024803
- DOI: 10.1093/braincomms/fcae095
Small fibre neuropathy in Fabry disease: a human-derived neuronal in vitro disease model and pilot data
Abstract
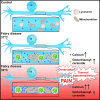
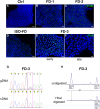
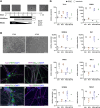
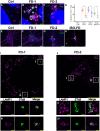
Acral burning pain triggered by fever, thermal hyposensitivity and skin denervation are hallmarks of small fibre neuropathy in Fabry disease, a life-threatening X-linked lysosomal storage disorder. Variants in the gene encoding alpha-galactosidase A may lead to impaired enzyme activity with cellular accumulation of globotriaosylceramide. To study the underlying pathomechanism of Fabry-associated small fibre neuropathy, we generated a neuronal in vitro disease model using patient-derived induced pluripotent stem cells from three Fabry patients and one healthy control. We further generated an isogenic control line via gene editing. We subjected induced pluripotent stem cells to targeted peripheral neuronal differentiation and observed intra-lysosomal globotriaosylceramide accumulations in somas and neurites of Fabry sensory neurons using super-resolution microscopy. At functional level, patch-clamp analysis revealed a hyperpolarizing shift of voltage-gated sodium channel steady-state inactivation kinetics in isogenic control neurons compared with healthy control neurons (P < 0.001). Moreover, we demonstrate a drastic increase in Fabry sensory neuron calcium levels at 39°C mimicking clinical fever (P < 0.001). This pathophysiological phenotype was accompanied by thinning of neurite calibres in sensory neurons differentiated from induced pluripotent stem cells derived from Fabry patients compared with healthy control cells (P < 0.001). Linear-nonlinear cascade models fit to spiking responses revealed that Fabry cell lines exhibit altered single neuron encoding properties relative to control. We further observed mitochondrial aggregation at sphingolipid accumulations within Fabry sensory neurites utilizing a click chemistry approach together with mitochondrial dysmorphism compared with healthy control cells. We pioneer pilot insights into the cellular mechanisms contributing to pain, thermal hyposensitivity and denervation in Fabry small fibre neuropathy and pave the way for further mechanistic in vitro studies in Fabry disease and the development of novel treatment approaches.
Keywords: Fabry disease; disease model; globotriaosylceramide; induced pluripotent stem cells; lysosomes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
References
-
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276(21):1163–1167. - PubMed
-
- Schiffmann R. Fabry disease. Pharmacol Ther. 2009;122(1):65–77. - PubMed
-
- Üçeyler N, Ganendiran S, Kramer D, Sommer C. Characterization of pain in Fabry disease. Clin J Pain. 2014;30(10):915–920. - PubMed
LinkOut - more resources
Full Text Sources
