Hi1a Improves Sensorimotor Deficit following Endothelin-1-Induced Stroke in Rats but Does Not Improve Functional Outcomes following Filament-Induced Stroke in Mice
- PMID: 38638162
- PMCID: PMC11022283
- DOI: 10.1021/acsptsci.3c00328
Hi1a Improves Sensorimotor Deficit following Endothelin-1-Induced Stroke in Rats but Does Not Improve Functional Outcomes following Filament-Induced Stroke in Mice
Abstract
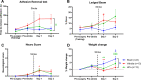
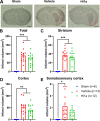
Activation of acid-sensing ion channel 1a (ASIC1a) plays a major role in mediating acidosis-induced neuronal injury following a stroke. Therefore, the inhibition of ASIC1a is a potential therapeutic avenue for the treatment of stroke. Venom-peptide Hi1a, a selective and highly potent ASIC1a inhibitor, reduces the infarct size and functional deficits when injected into the brain after stroke in rodents. However, its efficacy when administered using a clinically relevant route of administration remains to be established. Therefore, the current investigation aims to examine the efficacy of systemically administered Hi1a, using two different models of stroke in different species. Mice were subjected to the filament model of middle cerebral artery occlusion (MCAO) and treated with Hi1a systemically using either a single- or multiple-dosing regimen. 24 h poststroke, mice underwent functional testing, and the brain infarct size was assessed. Rats were subjected to endothelin-1 (ET-1)-induced MCAO and treated with Hi1a intravenously 2 h poststroke. Rats underwent functional tests prior to and for 3 days poststroke, when infarct volume was assessed. Mice receiving Hi1a did not show any improvements in functional outcomes, despite a trend toward reduced infarct size. This trend for reduced infarct size in mice was consistent regardless of the dosing regimen. There was also a trend toward lower infarct size in rats treated with Hi1a. More specifically, Hi1a reduced the amount of damage occurring within the somatosensory cortex, which was associated with an improved sensorimotor function in Hi1a-treated rats. Thus, this study suggests that Hi1a or more brain-permeable ASIC1a inhibitors are a potential stroke treatment.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Claudia A McCarthy, Robert E Widdop, Lachlan D Rash and Glenn F King are all inventors on a patent for Hi1a.
Figures


Similar articles
-
Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3750-3755. doi: 10.1073/pnas.1614728114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320941 Free PMC article.
-
Mechanism of acid-sensing ion channel modulation by Hi1a.J Gen Physiol. 2024 Dec 2;156(12):e202313519. doi: 10.1085/jgp.202313519. Epub 2024 Oct 24. J Gen Physiol. 2024. PMID: 39446054 Free PMC article.
-
PcTx1 affords neuroprotection in a conscious model of stroke in hypertensive rats via selective inhibition of ASIC1a.Neuropharmacology. 2015 Dec;99:650-7. doi: 10.1016/j.neuropharm.2015.08.040. Epub 2015 Aug 29. Neuropharmacology. 2015. PMID: 26320544
-
Acute inhibition of acid sensing ion channel 1a after spinal cord injury selectively affects excitatory synaptic transmission, but not intrinsic membrane properties, in deep dorsal horn interneurons.PLoS One. 2023 Nov 8;18(11):e0289053. doi: 10.1371/journal.pone.0289053. eCollection 2023. PLoS One. 2023. PMID: 37939057 Free PMC article.
-
Modeling Transient Focal Ischemic Stroke in Rodents by Intraluminal Filament Method of Middle Cerebral Artery Occlusion.Methods Mol Biol. 2018;1717:101-113. doi: 10.1007/978-1-4939-7526-6_9. Methods Mol Biol. 2018. PMID: 29468587 Review.
Cited by
-
A Comprehensive Review of the Pathophysiology of Neonatal Stroke and a Critique of Current and Future Therapeutic Strategies.Cells. 2025 Jun 16;14(12):910. doi: 10.3390/cells14120910. Cells. 2025. PMID: 40558537 Free PMC article. Review.
References
-
- World Health Organization ; Global Health Estimates 2019: Life expetancy and leading causes of death and disability; World Health Organization: Geneva; 2019. (cited 2021). Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-est....
-
- National Stroke Foundation ; National Stroke Audit Acute Services Report 2021; Stroke Foundation: Melbourne; 2022.
LinkOut - more resources
Full Text Sources
