TMC function, dysfunction, and restoration in mouse vestibular organs
- PMID: 38638308
- PMCID: PMC11024474
- DOI: 10.3389/fneur.2024.1356614
TMC function, dysfunction, and restoration in mouse vestibular organs
Abstract
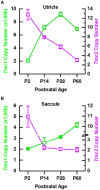
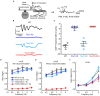
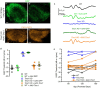
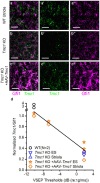
Tmc1 and Tmc2 are essential pore-forming subunits of mechanosensory transduction channels localized to the tips of stereovilli in auditory and vestibular hair cells of the inner ear. To investigate expression and function of Tmc1 and Tmc2 in vestibular organs, we used quantitative polymerase chain reaction (qPCR), fluorescence in situ hybridization - hairpin chain reaction (FISH-HCR), immunostaining, FM1-43 uptake and we measured vestibular evoked potentials (VsEPs) and vestibular ocular reflexes (VORs). We found that Tmc1 and Tmc2 showed dynamic developmental changes, differences in regional expression patterns, and overall expression levels which differed between the utricle and saccule. These underlying changes contributed to unanticipated phenotypic loss of VsEPs and VORs in Tmc1 KO mice. In contrast, Tmc2 KO mice retained VsEPs despite the loss of the calcium buffering protein calretinin, a characteristic biomarker of mature striolar calyx-only afferents. Lastly, we found that neonatal Tmc1 gene replacement therapy is sufficient to restore VsEP in Tmc1 KO mice for up to six months post-injection.
Keywords: TMC1; TMC2; hair cell; saccule; semicircular canal; utricle; vestibular.
Copyright © 2024 Ratzan, Lee, Madison, Zhu, Zhou, Géléoc and Holt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- World Health Organization (2020). Deafness and hearing loss. Available at: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss
-
- Shearer AE, Hildebrand MS, Schaefer AM, Smith RJH. Genetic hearing loss overview In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al., editors. GeneReviews®. Seattle, WA: University of Washington; (1993).
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

