Durable transgene expression and efficient re-administration after rAAV2.5T-mediated fCFTRΔR gene delivery to adult ferret lungs
- PMID: 38638546
- PMCID: PMC11024656
- DOI: 10.1016/j.omtm.2024.101244
Durable transgene expression and efficient re-administration after rAAV2.5T-mediated fCFTRΔR gene delivery to adult ferret lungs
Abstract
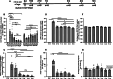
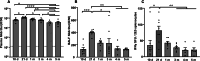
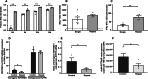
The dosing interval for effective recombinant adeno-associated virus (rAAV)-mediated gene therapy of cystic fibrosis lung disease remains unknown. Here, we assessed the durability of rAAV2.5T-fCFTRΔR-mediated transgene expression and neutralizing antibody (NAb) responses in lungs of adult wild-type ferrets. Within the first 3 months following rAAV2.5T-fCFTRΔR delivery to the lung, CFTRΔR transgene expression declined ∼5.6-fold and then remained stable to 5 months at ∼26% the level of endogenous CFTR. rAAV NAbs in the plasma and bronchoalveolar lavage fluid (BALF) peaked at 21 days, coinciding with peak ELISpot T cell responses to AAV capsid peptides, after which both responses declined and remained stable at 4-5 months post dosing. Administration of reporter vector rAAV2.5T-gLuc (gaussia luciferase) at 5 months following rAAV2.5T-fCFTRΔR dosing gave rise to similar levels of gLuc expression in the BALF as observed in age-matched reporter-only controls, demonstrating that residual BALF NAbs were functionally insignificant. Notably, the second vector administration led to a 2.6-fold greater ELISpot T cell response and ∼2.3-fold decline in fCFTRΔR mRNA and vector genomes derived from the initial rAAV2.5T-fCFTRΔR administration, suggesting selective destruction of transduced cells from the first vector dose. These findings provide insights into humoral and cellular immune response to rAAV that may be useful for optimizing gene therapy to the cystic fibrosis lung.
Keywords: CFTR; T cell responses; adeno-associated virus; cystic fibrosis; durability; gene therapy; lung; neutralizing antibodies; repeat administration; transgene.
© 2024 The Authors.
Conflict of interest statement
This work was funded by an SRA from Spirovant Sciences, Inc. Z.Y. and J.F.E. are consultants for Spirovant Sciences, Inc. K.E., M.P.L., M.S., and R.K. are employees of Spirovant Sciences, Inc.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

