Mitochondrial CypD Acetylation Promotes Endothelial Dysfunction and Hypertension
- PMID: 38639088
- PMCID: PMC11116043
- DOI: 10.1161/CIRCRESAHA.123.323596
Mitochondrial CypD Acetylation Promotes Endothelial Dysfunction and Hypertension
Abstract
Background: Nearly half of adults have hypertension, a major risk factor for cardiovascular disease. Mitochondrial hyperacetylation is linked to hypertension, but the role of acetylation of specific proteins is not clear. We hypothesized that acetylation of mitochondrial CypD (cyclophilin D) at K166 contributes to endothelial dysfunction and hypertension.
Methods: To test this hypothesis, we studied CypD acetylation in patients with essential hypertension, defined a pathogenic role of CypD acetylation in deacetylation mimetic CypD-K166R mutant mice and endothelial-specific GCN5L1 (general control of amino acid synthesis 5 like 1)-deficient mice using an Ang II (angiotensin II) model of hypertension.
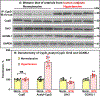
Results: Arterioles from hypertensive patients had 280% higher CypD acetylation coupled with reduced Sirt3 (sirtuin 3) and increased GCN5L1 levels. GCN5L1 regulates mitochondrial protein acetylation and promotes CypD acetylation, which is counteracted by mitochondrial deacetylase Sirt3. In human aortic endothelial cells, GCN5L1 depletion prevents superoxide overproduction. Deacetylation mimetic CypD-K166R mice were protected from vascular oxidative stress, endothelial dysfunction, and Ang II-induced hypertension. Ang II-induced hypertension increased mitochondrial GCN5L1 and reduced Sirt3 levels resulting in a 250% increase in GCN5L1/Sirt3 ratio promoting CypD acetylation. Treatment with mitochondria-targeted scavenger of cytotoxic isolevuglandins (mito2HOBA) normalized GCN5L1/Sirt3 ratio, reduced CypD acetylation, and attenuated hypertension. The role of mitochondrial acetyltransferase GCN5L1 in the endothelial function was tested in endothelial-specific GCN5L1 knockout mice. Depletion of endothelial GCN5L1 prevented Ang II-induced mitochondrial oxidative stress, reduced the maladaptive switch of vascular metabolism to glycolysis, prevented inactivation of endothelial nitric oxide, preserved endothelial-dependent relaxation, and attenuated hypertension.
Conclusions: These data support the pathogenic role of CypD acetylation in endothelial dysfunction and hypertension. We suggest that targeting cytotoxic mitochondrial isolevuglandins and GCN5L1 reduces CypD acetylation, which may be beneficial in cardiovascular disease.
Keywords: blood pressure; cardiovascular diseases; hypertension; mitochondria; superoxides.
Conflict of interest statement
Figures
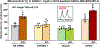

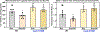


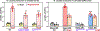
References
-
- Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997;28:1507–1517. - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

