Emergence of OXA-48-producing Klebsiella pneumoniae in Lithuania, 2023: a multi-cluster, multi-hospital outbreak
- PMID: 38639094
- PMCID: PMC11027475
- DOI: 10.2807/1560-7917.ES.2024.29.16.2400188
Emergence of OXA-48-producing Klebsiella pneumoniae in Lithuania, 2023: a multi-cluster, multi-hospital outbreak
Abstract
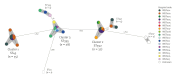
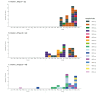
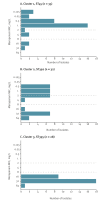
In 2023, an increase of OXA-48-producing Klebsiella pneumoniae was noticed by the Lithuanian National Public Health Surveillance Laboratory. Whole genome sequencing (WGS) of 106 OXA-48-producing K. pneumoniae isolates revealed three distinct clusters of carbapenemase-producing K. pneumoniae high-risk clones, including sequence type (ST) 45 (n = 35 isolates), ST392 (n = 32) and ST395 (n = 28), involving six, six and nine hospitals in different regions, respectively. These results enabled targeted investigation and control, and underscore the value of national WGS-based surveillance for antimicrobial resistance.
Keywords: Klebsiella pneumoniae; OXA-48; carbapenem-resistant Enterobacterales; carbapenemase; surveillance; whole genome sequencing.
Conflict of interest statement
Figures
References
-
- Ministry of Health of the Republic of Lithuania. Lietuvos Respublikos sveikatos apsaugos ministro įsakymas Nr. V-1194, priimtas 2013 m. gruodžio 18 d. Dėl Kliniškai ir epidemiologiškai svarbių mikroorganizmų atsparumo antimikrobiniams vaistams stebėsenos ir duomenų apie mikroorganizmų atsparumą antimikrobiniams vaistams rinkimo, kaupimo, analizės ir informacijos pateikimo tvarkos aprašo patvirtinimo. [Order No. V-1194 of the Minister of Health of the Republic of Lithuania of 18 December 2013. A description of procedures for the monitoring of antimicrobial resistance in clinically and epidemiologically relevant microorganisms and for the collection, compilation, analysis and reporting of antimicrobial resistance data]. Vilnius: Ministry of Health of the Republic of Lithuania; Register of Legal Acts. 31.12.2013: 2013-00188. Lithuanian. Available from: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.463410/dqbCGVGruC
-
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST clinical breakpoints - Version 14.0. Växjö: EUCAST; 2024. Available from: https://www.eucast.org/clinical_breakpoints
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
