Cetuximab chemotherapy resistance: Insight into the homeostatic evolution of head and neck cancer (Review)
- PMID: 38639184
- PMCID: PMC11056821
- DOI: 10.3892/or.2024.8739
Cetuximab chemotherapy resistance: Insight into the homeostatic evolution of head and neck cancer (Review)
Abstract
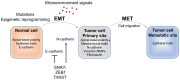
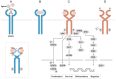
The complex evolution of genetic alterations in cancer that occurs in vivo is a selective process involving numerous factors and mechanisms. Chemotherapeutic agents that prevent the growth and spread of cancer cells induce selective pressure, leading to rapid artificial selection of resistant subclones. This rapid evolution is possible because antineoplastic drugs promote alterations in tumor‑cell metabolism, thus creating a bottleneck event. The few resistant cells that survive in this new environment obtain differential reproductive success that enables them to pass down the newly selected resistant gene pool. The present review aims to summarize key findings of tumor evolution, epithelial‑mesenchymal transition and resistance to cetuximab therapy in head and neck squamous cell carcinoma.
Keywords: cetuximab; drug resistance; epithelial‑mesenchymal transition; head and neck neoplasms; tumor evolution.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

