DANSPOT: A Multicenter Stepped-Wedge Cluster-Randomized Trial of the Reclassification of Acute Myocardial Infarction: Rationale and Study Design
- PMID: 38639348
- PMCID: PMC11179950
- DOI: 10.1161/JAHA.123.033493
DANSPOT: A Multicenter Stepped-Wedge Cluster-Randomized Trial of the Reclassification of Acute Myocardial Infarction: Rationale and Study Design
Abstract
Background: Cardiac troponins are the preferred biomarkers for the diagnosis of acute myocardial infarction. Although sex-specific 99th percentile thresholds of troponins are recommended in international guidelines, the clinical effect of their use is poorly investigated. The DANSPOT Study (The Danish Study of Sex- and Population-Specific 99th percentile upper reference limits of Troponin) aims to evaluate the clinical effect of a prospective implementation of population- and sex-specific diagnostic thresholds of troponins into clinical practice.
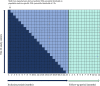
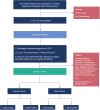
Methods: This study is a nationwide, multicenter, stepped-wedge cluster-randomized trial of the implementation of population- and sex-specific thresholds of troponins in 22 of 23 clinical centers in Denmark. We established sex-specific thresholds for 5 different troponin assays based on troponin levels in a healthy Danish reference population. Centers will sequentially cross over from current uniform manufacturer-derived thresholds to the new population- and sex-specific thresholds. The primary cohort is defined as patients with symptoms suggestive of acute coronary syndrome having at least 1 troponin measurement performed within 24 hours of arrival with a peak troponin value between the current uniform threshold and the new sex-specific female and male thresholds. The study will compare the occurrence of the primary outcome, defined as a composite of nonfatal myocardial infarction, unplanned revascularization, and all-cause mortality within 1 year, separately for men and women before and after the implementation of the new sex-specific thresholds.
Conclusions: The DANSPOT Study is expected to show the clinical effects on diagnostics, treatment, and clinical outcomes in patients with myocardial infarction of implementing sex-specific diagnostic thresholds for troponin based on a national Danish reference population.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT05336435.
Keywords: acute coronary syndrome; biomarkers; myocardial infarction; sex factors; troponin.
Figures

Similar articles
-
High-Sensitivity Cardiac Troponin-Optimizing the Diagnosis of Acute Myocardial Infarction/Injury in Women (CODE-MI): Rationale and design for a multicenter, stepped-wedge, cluster-randomized trial.Am Heart J. 2020 Nov;229:18-28. doi: 10.1016/j.ahj.2020.06.013. Epub 2020 Jun 25. Am Heart J. 2020. PMID: 32916606
-
High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial.Lancet. 2018 Sep 15;392(10151):919-928. doi: 10.1016/S0140-6736(18)31923-8. Epub 2018 Aug 28. Lancet. 2018. PMID: 30170853 Free PMC article. Clinical Trial.
-
Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction.Circulation. 2021 Aug 17;144(7):528-538. doi: 10.1161/CIRCULATIONAHA.121.054302. Epub 2021 Jun 25. Circulation. 2021. PMID: 34167318 Free PMC article.
-
Highly Sensitive Cardiac Troponins: The Evidence Behind Sex-Specific Cutoffs.J Am Heart Assoc. 2020 May 18;9(10):e015272. doi: 10.1161/JAHA.119.015272. Epub 2020 May 9. J Am Heart Assoc. 2020. PMID: 32390494 Free PMC article. Review.
-
High-sensitivity cardiac troponin assays: From improved analytical performance to enhanced risk stratification.Crit Rev Clin Lab Sci. 2017 May;54(3):143-172. doi: 10.1080/10408363.2017.1285268. Epub 2017 May 1. Crit Rev Clin Lab Sci. 2017. PMID: 28457177 Review.
Cited by
-
Second Year In Review, Third Year In Preview, and the 2024 JAHA Top 10.J Am Heart Assoc. 2025 Jan 7;14(1):e040732. doi: 10.1161/JAHA.124.040732. Epub 2025 Jan 7. J Am Heart Assoc. 2025. PMID: 39772643 Free PMC article. No abstract available.
References
-
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan G‐A, Dweck MR, Galbraith M, et al. 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2023;44:3720–3826. doi: 10.1093/eurheartj/ehad191 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

