ELUCIDATE Trial: A Single-Center Randomized Controlled Study
- PMID: 38639353
- PMCID: PMC11179944
- DOI: 10.1161/JAHA.123.033832
ELUCIDATE Trial: A Single-Center Randomized Controlled Study
Abstract
Background: Dapagliflozin, a sodium-glucose cotransporter 2 inhibitor, is an epochal oral antidiabetic drug that improves cardiorenal outcomes. However, the effect of early dapagliflozin intervention on left ventricular (LV) remodeling in patients with type 2 diabetes free from cardiovascular disease remains unclear.
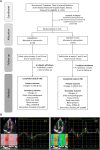
Methods and results: The ELUCIDATE trial was a prospective, open-label, randomized, active-controlled study that enrolled 76 patients with asymptomatic type 2 diabetes with LV ejection fraction ≥50%, randomized to the dapagliflozin 10 mg/day add-on or standard-of-care group. Speckle-tracking echocardiography-based measurements of the cardiac global longitudinal strain were performed at baseline and 24 weeks after treatment initiation. Patients who received dapagliflozin had a greater reduction in LV dimension (1.68 mm [95% CI, 0.53-2.84]; P=0.005), LV end-systolic volume (5.51 mL [95% CI, 0.86-10.17]; P=0.021), and LV mass index (4.25 g/m2.7 [95% CI, 2.42-6.09]; P<0.0001) compared with standard of care in absolute mean differences. Dapagliflozin add-on therapy led to a significant LV global longitudinal strain increment (0.74% [95% CI, 1.00-0.49]; P<0.0001) and improved LV systolic and early diastolic strain rates (0.27/s [95% CI, 0.17-0.60]; and 0.11/s [95% CI, 0.06-0.16], respectively; both P<0.0001) but not in global circumferential strain. No significant changes were found in insulin resistance, NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels, or other biomarkers at 6 months after the dapagliflozin administration.
Conclusions: Dapagliflozin add-on therapy could lead to more favorable cardiac remodeling accompanied by enhanced cardiac mechanical function among patients with asymptomatic type 2 diabetes. Our findings provide evidence of the efficacy of dapagliflozin use for the primary prevention of diabetic cardiomyopathy.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03871621.
Keywords: clinical trial; dapagliflozin; echocardiography; sodium–glucose cotransporter 2 inhibitors; type 2 diabetes; ventricular remodeling.
Figures


References
-
- Action to Control Cardiovascular Risk in Diabetes Study G , Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Beigi FI, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743 - DOI - PMC - PubMed
-
- Faden G, Faganello G, De Feo S, Berlinghieri N, Tarantini L, Lenarda AD, Faggiano P, Cioffi G. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: data from the SHORTWAVE study. Diabetes Res Clin Pract. 2013;101:309–316. doi: 10.1016/j.diabres.2013.07.004 - DOI - PubMed
-
- Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the atherosclerosis risk in the community study. Circ Heart Fail. 2015;8:448–454. doi: 10.1161/CIRCHEARTFAILURE.114.001990 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

