Stewardship Prompts to Improve Antibiotic Selection for Urinary Tract Infection: The INSPIRE Randomized Clinical Trial
- PMID: 38639723
- PMCID: PMC11185978
- DOI: 10.1001/jama.2024.6259
Stewardship Prompts to Improve Antibiotic Selection for Urinary Tract Infection: The INSPIRE Randomized Clinical Trial
Abstract
Importance: Urinary tract infection (UTI) is the second most common infection leading to hospitalization and is often associated with gram-negative multidrug-resistant organisms (MDROs). Clinicians overuse extended-spectrum antibiotics although most patients are at low risk for MDRO infection. Safe strategies to limit overuse of empiric antibiotics are needed.
Objective: To evaluate whether computerized provider order entry (CPOE) prompts providing patient- and pathogen-specific MDRO risk estimates could reduce use of empiric extended-spectrum antibiotics for treatment of UTI.
Design, setting, and participants: Cluster-randomized trial in 59 US community hospitals comparing the effect of a CPOE stewardship bundle (education, feedback, and real-time and risk-based CPOE prompts; 29 hospitals) vs routine stewardship (n = 30 hospitals) on antibiotic selection during the first 3 hospital days (empiric period) in noncritically ill adults (≥18 years) hospitalized with UTI with an 18-month baseline (April 1, 2017-September 30, 2018) and 15-month intervention period (April 1, 2019-June 30, 2020).
Interventions: CPOE prompts recommending empiric standard-spectrum antibiotics in patients ordered to receive extended-spectrum antibiotics who have low estimated absolute risk (<10%) of MDRO UTI, coupled with feedback and education.
Main outcomes and measures: The primary outcome was empiric (first 3 days of hospitalization) extended-spectrum antibiotic days of therapy. Secondary outcomes included empiric vancomycin and antipseudomonal days of therapy. Safety outcomes included days to intensive care unit (ICU) transfer and hospital length of stay. Outcomes were assessed using generalized linear mixed-effect models to assess differences between the baseline and intervention periods.
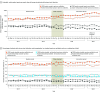
Results: Among 127 403 adult patients (71 991 baseline and 55 412 intervention period) admitted with UTI in 59 hospitals, the mean (SD) age was 69.4 (17.9) years, 30.5% were male, and the median Elixhauser Comorbidity Index count was 4 (IQR, 2-5). Compared with routine stewardship, the group using CPOE prompts had a 17.4% (95% CI, 11.2%-23.2%) reduction in empiric extended-spectrum days of therapy (rate ratio, 0.83 [95% CI, 0.77-0.89]; P < .001). The safety outcomes of mean days to ICU transfer (6.6 vs 7.0 days) and hospital length of stay (6.3 vs 6.5 days) did not differ significantly between the routine and intervention groups, respectively.
Conclusions and relevance: Compared with routine stewardship, CPOE prompts providing real-time recommendations for standard-spectrum antibiotics for patients with low MDRO risk coupled with feedback and education significantly reduced empiric extended-spectrum antibiotic use among noncritically ill adults admitted with UTI without changing hospital length of stay or days to ICU transfers.
Trial registration: ClinicalTrials.gov Identifier: NCT03697096.
Conflict of interest statement
Figures

Comment in
-
Harnessing the Electronic Health Record to Improve Empiric Antibiotic Prescribing.JAMA. 2024 Jun 18;331(23):1993-1994. doi: 10.1001/jama.2024.6554. JAMA. 2024. PMID: 38639731 No abstract available.
-
A prompt-based stewardship to reduce extended-spectrum antibiotic treatment in UTI.Nat Rev Urol. 2024 Jul;21(7):390. doi: 10.1038/s41585-024-00908-5. Nat Rev Urol. 2024. PMID: 38872011 No abstract available.
-
In adult inpatients with a UTI, CPOE-based vs. routine stewardship reduced extended-spectrum antibiotic use.Ann Intern Med. 2024 Aug;177(8):JC91. doi: 10.7326/ANNALS-24-00972-JC. Epub 2024 Aug 6. Ann Intern Med. 2024. PMID: 39102727
References
-
- Magill SS, O’Leary E, Ray SM, et al. ; Emerging Infections Program Hospital Prevalence Survey Team . Antimicrobial use in US hospitals: comparison of results from Emerging Infections Program prevalence surveys, 2015 and 2011. Clin Infect Dis. 2021;72(10):1784-1792. doi: 10.1093/cid/ciaa373 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019. Accessed September 16, 2023. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re...

