Replacing Animal Testing with Stem Cell-Organoids : Advantages and Limitations
- PMID: 38639829
- PMCID: PMC11319430
- DOI: 10.1007/s12015-024-10723-5
Replacing Animal Testing with Stem Cell-Organoids : Advantages and Limitations
Abstract
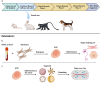
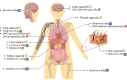
Various groups including animal protection organizations, medical organizations, research centers, and even federal agencies such as the U.S. Food and Drug Administration, are working to minimize animal use in scientific experiments. This movement primarily stems from animal welfare and ethical concerns. However, recent advances in technology and new studies in medicine have contributed to an increase in animal experiments throughout the years. With the rapid increase in animal testing, concerns arise including ethical issues, high cost, complex procedures, and potential inaccuracies.Alternative solutions have recently been investigated to address the problems of animal testing. Some of these technologies are related to stem cell technologies, such as organ-on-a-chip, organoids, and induced pluripotent stem cell models. The aim of the review is to focus on stem cell related methodologies, such as organoids, that can serve as an alternative to animal testing and discuss its advantages and limitations, alongside regulatory considerations.Although stem cell related methodologies has shortcomings, it has potential to replace animal testing. Achieving this requires further research on stem cells, with potential societal and technological benefits.
Keywords: Animal Testing Alternatives; Animal Testing law; Organ-on-chips; Organoids; Stem Cell; iPSC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

