NFATc4 Knockout Promotes Neuroprotection and Retinal Ganglion Cell Regeneration After Optic Nerve Injury
- PMID: 38639863
- PMCID: PMC11496353
- DOI: 10.1007/s12035-024-04129-0
NFATc4 Knockout Promotes Neuroprotection and Retinal Ganglion Cell Regeneration After Optic Nerve Injury
Abstract
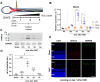
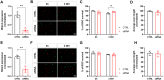
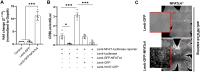
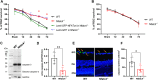
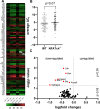
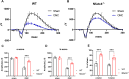
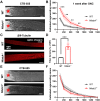
Retinal ganglion cells (RGCs), neurons transmitting visual information via the optic nerve, fail to regenerate their axons after injury. The progressive loss of RGC function underlies the pathophysiology of glaucoma and other optic neuropathies, often leading to irreversible blindness. Therefore, there is an urgent need to identify the regulators of RGC survival and the regenerative program. In this study, we investigated the role of the family of transcription factors known as nuclear factor of activated T cells (NFAT), which are expressed in the retina; however, their role in RGC survival after injury is unknown. Using the optic nerve crush (ONC) model, widely employed to study optic neuropathies and central nervous system axon injury, we found that NFATc4 is specifically but transiently up-regulated in response to mechanical injury. In the injured retina, NFATc4 immunolocalized primarily to the ganglionic cell layer. Utilizing NFATc4-/- and NFATc3-/- mice, we demonstrated that NFATc4, but not NFATc3, knockout increased RGC survival, improved retina function, and delayed axonal degeneration. Microarray screening data, along with decreased immunostaining of cleaved caspase-3, revealed that NFATc4 knockout was protective against ONC-induced degeneration by suppressing pro-apoptotic signaling. Finally, we used lentiviral-mediated NFATc4 delivery to the retina of NFATc4-/- mice and reversed the pro-survival effect of NFATc4 knockout, conclusively linking the enhanced survival of injured RGCs to NFATc4-dependent mechanisms. In summary, this study is the first to demonstrate that NFATc4 knockout may confer transient RGC neuroprotection and decelerate axonal degeneration after injury, providing a potent therapeutic strategy for optic neuropathies.
Keywords: Apoptotic gene expression; Intravitreal gene delivery; NFATc4 transcription factor; Optic nerve injury; Retinal ganglion cell survival.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regenerative Responses and Axon Pathfinding of Retinal Ganglion Cells in Chronically Injured Mice.Invest Ophthalmol Vis Sci. 2017 Mar 1;58(3):1743-1750. doi: 10.1167/iovs.16-19873. Invest Ophthalmol Vis Sci. 2017. PMID: 28324115 Free PMC article.
-
Role of SARM1 and DR6 in retinal ganglion cell axonal and somal degeneration following axonal injury.Exp Eye Res. 2018 Jun;171:54-61. doi: 10.1016/j.exer.2018.03.007. Epub 2018 Mar 8. Exp Eye Res. 2018. PMID: 29526794 Free PMC article.
-
Absence of galectin-3 promotes neuroprotection in retinal ganglion cells after optic nerve injury.Histol Histopathol. 2017 Mar;32(3):253-262. doi: 10.14670/HH-11-788. Epub 2016 Jun 3. Histol Histopathol. 2017. PMID: 27255346
-
Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy.Front Immunol. 2022 Mar 4;13:860070. doi: 10.3389/fimmu.2022.860070. eCollection 2022. Front Immunol. 2022. PMID: 35309305 Free PMC article. Review.
-
Evaluating the Evidence for Neuroprotective and Axonal Regenerative Activities of Different Inflammatory Cell Types After Optic Nerve Injury.Mol Neurobiol. 2025 May;62(5):6212-6227. doi: 10.1007/s12035-024-04679-3. Epub 2024 Dec 30. Mol Neurobiol. 2025. PMID: 39738875 Free PMC article. Review.
Cited by
-
Neuroprotective Effect of Methylene Blue in a Rat Model of Traumatic Optic Neuropathy.Pharmaceuticals (Basel). 2025 Jun 19;18(6):920. doi: 10.3390/ph18060920. Pharmaceuticals (Basel). 2025. PMID: 40573315 Free PMC article.
-
Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies.Pharmaceuticals (Basel). 2024 Sep 25;17(10):1261. doi: 10.3390/ph17101261. Pharmaceuticals (Basel). 2024. PMID: 39458902 Free PMC article. Review.
-
Perinuclear compartment controls calcineurin/MEF2 signaling for axonal outgrowth of hippocampal neurons.Front Mol Neurosci. 2024 Nov 25;17:1494160. doi: 10.3389/fnmol.2024.1494160. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39654556 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

