Multicenter, phase 1 study of etavopivat (FT-4202) treatment for up to 12 weeks in patients with sickle cell disease
- PMID: 38640200
- PMCID: PMC11445223
- DOI: 10.1182/bloodadvances.2023012467
Multicenter, phase 1 study of etavopivat (FT-4202) treatment for up to 12 weeks in patients with sickle cell disease
Abstract
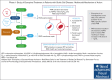
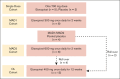
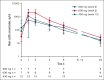
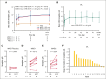
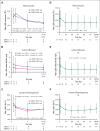
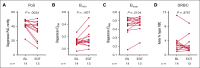
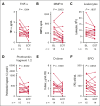
Etavopivat is an investigational, once daily, oral, selective erythrocyte pyruvate kinase (PKR) activator. A multicenter, randomized, placebo-controlled, double-blind, 3-part, phase 1 study was conducted to characterize the safety and clinical activity of etavopivat. Thirty-six patients with sickle cell disease (SCD) were enrolled into 4 cohorts: 1 single-dose, 2 multiple ascending doses, and 1 open-label (OL). In the OL cohort, 15 patients (median age 33.0 years [range, 17-55]) received 400 mg etavopivat once daily for 12 weeks; 14 patients completed treatment. Consistent with the mechanism of PKR activation, increases in adenosine triphosphate and decreases in 2,3-diphosphoglycerate were observed and sustained over 12 weeks' treatment. This translated clinically to an increase in hemoglobin (Hb; mean maximal increase 1.6 g/dL [range, 0.8-2.8]), with >1 g/dL increase in 11 (73%) patients during treatment. In addition, the oxygen tension at which Hb is 50% saturated was reduced (P = .0007) with a concomitant shift in point of sickling (P = .0034) to lower oxygen tension in oxygen-gradient ektacytometry. Hemolysis markers (absolute reticulocyte count, indirect bilirubin, and lactate dehydrogenase) decreased from baseline, along with matrix metalloproteinase-9 and erythropoietin. In the OL cohort, adverse events (AEs) were mostly grade 1/2, consistent with underlying SCD; 5 patients had serious AEs. Vaso-occlusive pain episode was the most common treatment-emergent AE (n = 7) in the OL cohort. In this, to our knowledge, the first study of etavopivat in SCD, 400 mg once daily for 12 weeks was well tolerated, resulting in rapid and sustained increases in Hb, improved red blood cell physiology, and decreased hemolysis. This trial was registered at www.ClinicalTrials.gov as #NCT03815695.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.W., S.F., J.G., I.O., P.S., and M.R. were employees of and held stock in Forma Therapeutics, Inc, which was acquired by Novo Nordisk, at the time of this study. P.F.K was a consultant to Forma Therapeutics, Inc, which was acquired by Novo Nordisk, was an employee when the study was performed, and held stock in Forma Therapeutic, Inc. I.O. is a former principal investigator at Levine Cancer Institute/Atrium health and formerly held consultancies with Forma Therapeutics, Novo Nordisk, Agios, Global Blood Therapeutics (GBT), Novartis, Cheisi, Acceleron, and Emmaus; served on speaker’s bureau for Novartis and GBT; received research funding from the Centers for Disease Control, Health Resources and Service’s Administration, and Patient Centered Outcomes Research Institute; and was an employee of Forma Therapeutics between February and October 2022 and is currently an employee of Novo Nordisk since October 2022. S.L.S. reports consultancies with Forma Therapeutics, Inc, Novo Nordisk, GBT/Pfizer, ORIC Pharmaceuticals, Agios, and Beam Therapeutics; membership on the advisory committees of GBT/Pfizer and Novartis; and research funding from Forma Therapeutics, Inc, Novo Nordisk, GBT, Novartis, and Pfizer. R.H. reports consultancies with Bristol Myers Squib, GBT, Imara, National Institutes of Health (NIH), Novartis and research funding from Chiesi, Forma Therapeutics, Inc, and the University of Pittsburgh. M.I. reports consultancies with GBT; receives research funding from Novartis, Pfizer, GBT, Agios, Alexion, Novo Nordisk, and Forma; serves on the GBT speaker’s bureau; and is a member of the board/advisory committee of GBT. R.C.B. was a principal investigator at Children’s Healthcare of Atlanta at the time of study and formally held consultancies with GBT, Imara, Novartis; received funding from GBT, Novartis, Forma Therapeutics, and Imara; and has been an employee of GBT, a wholly owned subsidiary of Pfizer as of October 2023, since July 2022. F.A.K. received research funding from Forma. T.A.K. reports consultancies, membership on advisory boards, and research funding from Forma Therapeutics, Inc, Novo Nordisk, Agios Pharmaceuticals, Inc; and research funding from the NIH. M.J.T. reports consultancies with GlycoMimetics, Inc; served on a data safety monitoring board of Novartis; and received research funding from Forma Therapeutics, Inc, CSL Behring, Inc, Doris Duke Charitable Foundation, and the NIH. K.C. declares no competing financial interests.
Figures

Comment in
-
Doubling down on PK activation for sickle cell disease.Blood Adv. 2024 Aug 27;8(16):4457-4458. doi: 10.1182/bloodadvances.2024013367. Blood Adv. 2024. PMID: 39190327 Free PMC article. No abstract available.
References
-
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4 - PubMed
-
- Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. - PubMed
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. - PubMed
-
- Kapoor S, Little JA, Pecker LH. Advances in the treatment of sickle cell disease. Mayo Clin Proc. 2018;93(12):1810–1824. - PubMed

