From 13C-lignin to 13C-mycelium: Agaricus bisporus uses polymeric lignin as a carbon source
- PMID: 38640242
- PMCID: PMC11029805
- DOI: 10.1126/sciadv.adl3419
From 13C-lignin to 13C-mycelium: Agaricus bisporus uses polymeric lignin as a carbon source
Abstract
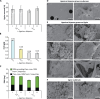
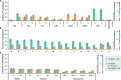
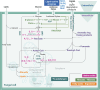
Plant biomass conversion by saprotrophic fungi plays a pivotal role in terrestrial carbon (C) cycling. The general consensus is that fungi metabolize carbohydrates, while lignin is only degraded and mineralized to CO2. Recent research, however, demonstrated fungal conversion of 13C-monoaromatic compounds into proteinogenic amino acids. To unambiguously prove that polymeric lignin is not merely degraded, but also metabolized, carefully isolated 13C-labeled lignin served as substrate for Agaricus bisporus, the world's most consumed mushroom. The fungus formed a dense mycelial network, secreted lignin-active enzymes, depolymerized, and removed lignin. With a lignin carbon use efficiency of 0.14 (g/g) and fungal biomass enrichment in 13C, we demonstrate that A. bisporus assimilated and further metabolized lignin when offered as C-source. Amino acids were high in 13C-enrichment, while fungal-derived carbohydrates, fatty acids, and ergosterol showed traces of 13C. These results hint at lignin conversion via aromatic ring-cleaved intermediates to central metabolites, underlining lignin's metabolic value for fungi.
Figures


References
-
- Liang C., Schimel J. P., Jastrow J. D., The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 17105 (2017). - PubMed
-
- Tao F., Huang Y., Hungate B. A., Manzoni S., Frey S. D., Schmidt M. W. I., Reichstein M., Carvalhais N., Ciais P., Jiang L., Lehmann J., Wang Y. P., Houlton B. Z., Ahrens B., Mishra U., Hugelius G., Hocking T. D., Lu X., Shi Z., Viatkin K., Vargas R., Yigini Y., Omuto C., Malik A. A., Peralta G., Cuevas-Corona R., Di Paolo L. E., Luotto I., Liao C., Liang Y. S., Saynes V. S., Huang X., Luo Y., Microbial carbon use efficiency promotes global soil carbon storage. Nature 618, 981–985 (2023). - PMC - PubMed
-
- Cragg S. M., Beckham G. T., Bruce N. C., Bugg T. D. H., Distel D. L., Dupree P., Etxabe A. G., Goodell B. S., Jellison J., McGeehan J. E., McQueen-Mason S. J., Schnorr K., Walton P. H., Watts J. E. M., Zimmer M., Lignocellulose degradation mechanisms across the tree of life. Curr. Opin. Chem. Biol. 29, 108–119 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

