Liver-targeted polymeric prodrugs delivered subcutaneously improve tafenoquine therapeutic window for malaria radical cure
- PMID: 38640243
- PMCID: PMC11029812
- DOI: 10.1126/sciadv.adk4492
Liver-targeted polymeric prodrugs delivered subcutaneously improve tafenoquine therapeutic window for malaria radical cure
Abstract
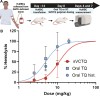
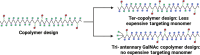
Approximately 3.3 billion people live with the threat of Plasmodium vivax malaria. Infection can result in liver-localized hypnozoites, which when reactivated cause relapsing malaria. This work demonstrates that an enzyme-cleavable polymeric prodrug of tafenoquine addresses key requirements for a mass administration, eradication campaign: excellent subcutaneous bioavailability, complete parasite control after a single dose, improved therapeutic window compared to the parent oral drug, and low cost of goods sold (COGS) at less than $1.50 per dose. Liver targeting and subcutaneous dosing resulted in improved liver:plasma exposure profiles, with increased efficacy and reduced glucose 6-phosphate dehydrogenase-dependent hemotoxicity in validated preclinical models. A COGS and manufacturability analysis demonstrated global scalability, affordability, and the ability to redesign this fully synthetic polymeric prodrug specifically to increase global equity and access. Together, this polymer prodrug platform is a candidate for evaluation in human patients and shows potential for P. vivax eradication campaigns.
Figures




References
-
- C. Kimeu, “Eliminate malaria once and for all or it will come back stronger, UN warned,” The Guardian, 22 September 2023; https://theguardian.com/global-development/2023/sep/22/eliminate-malaria....
-
- Global Malaria Programme, World Health Organization, World Malaria Report 2022; https://who.int/teams/global-malaria-programme/reports/world-malaria-rep....
-
- Battle K. E., Lucas T. C. D., Nguyen M., Howes R. E., Nandi A. K., Twohig K. A., Pfeffer D. A., Cameron E., Rao P. C., Casey D., Gibson H. S., Rozier J. A., Dalrymple U., Keddie S. H., Collins E. L., Harris J. R., Guerra C. A., Thorn M. P., Bisanzio D., Fullman N., Huynh C. K., Kulikoff X., Kutz M. J., Lopez A. D., Mokdad A. H., Naghavi M., Nguyen G., Shackelford K. A., Vos T., Wang H., Lim S. S., Murray C. J. L., Price R. N., Baird J. K., Smith D. L., Bhatt S., Weiss D. J., Hay S. I., Gething P. W., Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000-17: A spatial and temporal modelling study. Lancet 394, 332–343 (2019). - PMC - PubMed
-
- Hundessa S., Williams G., Li S., Liu L., Cao W., Ren H., Guo J., Gasparrini A., Ebi K., Zhang W., Guo Y., Projecting potential spatial and temporal changes in the distribution of Plasmodium vivax and Plasmodium falciparum malaria in China with climate change. Sci. Total Environ. 627, 1285–1293 (2018). - PMC - PubMed
-
- Trájer A. J., The changing risk patterns of Plasmodium vivax malaria in Greece due to climate change. Int. J. Environ. Health Res. 32, 665–690 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

