Efficacy and safety of Buyang Huanwu Decoction in patients with spinal cord injury: A meta-analysis of randomized controlled trials
- PMID: 38640259
- PMCID: PMC11030014
- DOI: 10.1097/MD.0000000000037865
Efficacy and safety of Buyang Huanwu Decoction in patients with spinal cord injury: A meta-analysis of randomized controlled trials
Abstract
Background: There has been growing interest in using the traditional Chinese herb Buyang Huanwu Decoction (BHD) as a potential treatment for spinal cord injury (SCI), owing to its long-used treatment for SCI in China. However, the efficacy and safety of BHD treatment for SCI remain widely skeptical. This meta-analysis aims to assess the safety and efficacy of BHD in managing SCI.
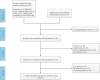
Method: A comprehensive literature search was conducted across several databases, including PubMed, EMBASE, Cochrane Library, CNKI, Wanfang, VIP, and Sinomed, up to January 1, 2024. Randomized controlled clinical trials evaluating the safety or efficacy of BHD in SCI treatment were included. The analysis focused on 8 critical endpoints: Patient-perceived total clinical effective rate, American Spinal Cord Injury Association (ASIA) sensory score, ASIA motor score, somatosensory evoked potential, motor evoked potential, visual analog scale pain score, Japanese Orthopaedic Association score, and adverse events.
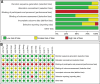
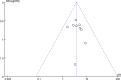
Results: Thirteen studies comprising 815 participants met the inclusion criteria. No significant heterogeneity or publication bias was observed across the trials. The findings revealed significant improvements in the patient-perceived total clinical effective rate (OR = 3.77; 95% confidence interval [CI] = [2.43, 5.86]; P < .001), ASIA sensory score (mean difference [MD] = 8.22; 95% CI = [5.87, 10.56]; P < .001), ASIA motor score (MD = 7.16; 95% CI = [5.15, 9.18]; P < .001), somatosensory evoked potential (MD = 0.25; 95% CI = [0.03, 0.48]; P = .02), motor evoked potential (MD = 0.30; 95% CI = [0.14, 0.46]; P = .0002), and Japanese Orthopaedic Association score (MD = 1.99; 95% CI = [0.39, 3.58]; P = .01) in the BHD combination group compared to the control group. Additionally, there was a significant reduction in visual analog scale pain scores (MD = -0.81; 95% CI = [-1.52, -0.11]; P = .02) with BHD combination treatment, without a significant increase in adverse effects (OR = 0.68; 95% CI = [0.33, 1.41]; P = .3).
Conclusion: The current evidence suggests that BHD is effective and safe in treating SCI, warranting consideration as a complementary and alternative therapy. However, given the low methodological quality of the included studies, further rigorous research is warranted to validate these findings.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Chinese herbal medicine Buyang Huanwu Decoction in treatment of peripheral nerve injury: A systematic review and meta-analysis of randomized controlled trials.Medicine (Baltimore). 2023 Jul 21;102(29):e34256. doi: 10.1097/MD.0000000000034256. Medicine (Baltimore). 2023. PMID: 37478277 Free PMC article.
-
Chinese Herbal Medicine in Treatment of Spinal Cord Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Am J Chin Med. 2020;48(7):1593-1616. doi: 10.1142/S0192415X20500792. Epub 2020 Nov 5. Am J Chin Med. 2020. PMID: 33148008
-
[Efficacy and safety of Buyang Huanwu Decoction for early-stage diabetic nephropathy: a Meta-analysis].Zhongguo Zhong Yao Za Zhi. 2019 Apr;44(8):1660-1667. doi: 10.19540/j.cnki.cjcmm.20190109.001. Zhongguo Zhong Yao Za Zhi. 2019. PMID: 31090332 Chinese.
-
Effect of Buyang Huanwu decoction for the rehabilitation of ischemic stroke patients: a meta-analysis of randomized controlled trials.Health Qual Life Outcomes. 2021 Mar 9;19(1):79. doi: 10.1186/s12955-021-01728-6. Health Qual Life Outcomes. 2021. PMID: 33750396 Free PMC article. Review.
-
Chinese herbal medicine Buyang Huanwu Decoction combined with acupuncture to treat sequela of apoplexy: a meta-analysis of randomized controlled trials.Ann Palliat Med. 2021 Feb;10(2):1685-1692. doi: 10.21037/apm-20-1092. Epub 2020 Nov 18. Ann Palliat Med. 2021. PMID: 33222469
Cited by
-
Global research hotspots and trends of Buyang huanwu decoction: A visual analysis of the literature based on CiteSpace.Medicine (Baltimore). 2024 Nov 8;103(45):e40457. doi: 10.1097/MD.0000000000040457. Medicine (Baltimore). 2024. PMID: 39533635 Free PMC article.
References
-
- Witiw CD, Fehlings MG. Acute spinal cord injury. J Spinal Disord Tech. 2015;28:202–10. - PubMed
-
- Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25:898–908. - PubMed
-
- Sharif S, Jazaib Ali MY. Outcome prediction in spinal cord injury: myth or reality. World Neurosurg. 2020;140:574–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

