Evaluating the efficacy and safety of Alzheimer's disease drugs: A meta-analysis and systematic review
- PMID: 38640313
- PMCID: PMC11029996
- DOI: 10.1097/MD.0000000000037799
Evaluating the efficacy and safety of Alzheimer's disease drugs: A meta-analysis and systematic review
Abstract
Background: Alzheimer's disease (AD) is a progressive neurodegenerative disorder. Dementia severity was assessed mainly through cognitive function, psychobehavioral symptoms, and daily living ability. Currently, there are not many drugs that can be selected to treat mild to moderate AD, and the value of drugs remains controversial.
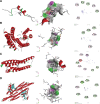
Objective: The aim of this study is to quantitatively evaluate the efficacy and safety of cholinesterase inhibitors (ChEIs), memantine, and sodium oligomannate (GV-971) in the treatment of patients with AD. Additionally, molecular docking analysis will be used to investigate the binding affinities of donepezil, galantamine, rivastigmine, and memantine with key receptor proteins associated with AD, including beta-amyloid (Abeta), microtubule-associated protein (MAP), apolipoprotein E4 (APOE4), and Mitofusin-2 (MFN2), to further validate the results of the meta-analysis.
Methods: We obtained clinical trials characterized by randomization, placebo control, and double-blinded methodologies concerning ChEIs, memantine, and GV-971. Statistical analysis was performed using Review Manager Version 5.4 software. Molecular docking was also conducted to evaluate the results.
Results: All drugs improved the cognitive function, with the effect value ranging from -1.23 (95% CI -2.17 to -0.30) for 20 mg memantine to -3.29 (95% CI -4.14 to -2.45) for 32 mg galantamine. Although 32 mg galanthamine and GV-971 did not improve the clinicians' Global Impression of Change scale, other drugs showed significant results compared with placebo. On NPI, only 10 mg of donepezil and 24 mg of galantamine had improvement effects. On ADCS/ADL, only 20 mg memantine and 900 mg GV-971 had no significant difference from the placebo. Donepezil 5 mg and GV-971 900 mg did not increase the drug withdrawal rates due to various reasons or adverse reactions when compared to the placebo. Donepezil demonstrated superior binding to the protein and exhibited greater efficacy compared to other drugs.
Conclusion: ChEIs, memantine, and GV-971 all can slow the progression of AD but have different effects on respective assessments. Donepezil and GV-971 were relatively well tolerated.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures








References
-
- Asher S, Priefer R. Alzheimer’s disease failed clinical trials. Life Sci. 2022;306:120861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

