Particulate bioaerogels for respiratory drug delivery
- PMID: 38641021
- PMCID: PMC11847494
- DOI: 10.1016/j.jconrel.2024.04.021
Particulate bioaerogels for respiratory drug delivery
Abstract
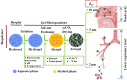
The bioaerogel microparticles have been recently developed for respiratory drug delivery and attract fast increasing interests. These highly porous microparticles have ultralow density and hence possess much reduced aerodynamic diameter, which favour them with greatly enhanced dispersibility and improved aerosolisation behaviour. The adjustable particle geometric dimensions by varying preparation methods and controlling operation parameters make it possible to fabricate bioaerogel microparticles with accurate sizes for efficient delivery to the targeted regions of respiratory tract (i.e. intranasal and pulmonary). Additionally, the technical process can provide bioaerogel microparticles with the opportunities of accommodating polar, weak polar and non-polar drugs at sufficient amount to satisfy clinical needs, and the adsorbed drugs are primarily in the amorphous form that potentially can facilitate drug dissolution and improve bioavailability. Finally, the nature of biopolymers can further offer additional advantageous characteristics of improved mucoadhesion, sustained drug release and subsequently elongated time for continuous treatment on-site. These fascinating features strongly support bioaerogel microparticles to become a novel platform for effective delivery of a wide range of drugs to the targeted respiratory regions, with increased drug residence time on-site, sustained drug release, constant treatment for local and systemic diseases and anticipated better-quality of therapeutic effects.
Keywords: Amorphous form; Bioaerogel particulate platform; Improved aerosolisation performance; Targeted respiratory drug delivery; Ultralow densities.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Claus S., Weiler C., Schiewe J., Friess W. How can we bring high drug doses to the lung. Eur. J. Pharm. Biopharm. 2014;86:1–6. - PubMed
-
- de Boer A.H., Hagedoorn P., Hoppentocht M., Buttini F., Grasmeijer F., Frijlink H.W. Dry powder inhalation: past, present and future. Expert Opin. Drug Deliv. 2017;14:499–512. - PubMed
-
- Momin M.A.M., Tucker I.G., Das S.C. High dose dry powder inhalers to overcome the challenges of tuberculosis treatment. Int. J. Pharm. 2018;550:398–417. - PubMed
-
- Newman S.P. Dry powder inhalers for optimal drug delivery. Expert Opin. Biol. Ther. 2005;4:23–33. - PubMed
-
- Prime D., Atkins P.J., Slater A., Sumby B. Review of dry powder inhalers. Adv. Drug Deliv. Rev. 1997;26:51–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

