The multifaceted role of intracellular glycosylation in cytoprotection and heart disease
- PMID: 38641064
- PMCID: PMC11126959
- DOI: 10.1016/j.jbc.2024.107296
The multifaceted role of intracellular glycosylation in cytoprotection and heart disease
Abstract
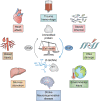
The modification of nuclear, cytoplasmic, and mitochondrial proteins by O-linked β-N-actylglucosamine (O-GlcNAc) is an essential posttranslational modification that is common in metozoans. O-GlcNAc is cycled on and off proteins in response to environmental and physiological stimuli impacting protein function, which, in turn, tunes pathways that include transcription, translation, proteostasis, signal transduction, and metabolism. One class of stimulus that induces rapid and dynamic changes to O-GlcNAc is cellular injury, resulting from environmental stress (for instance, heat shock), hypoxia/reoxygenation injury, ischemia reperfusion injury (heart attack, stroke, trauma hemorrhage), and sepsis. Acute elevation of O-GlcNAc before or after injury reduces apoptosis and necrosis, suggesting that injury-induced changes in O-GlcNAcylation regulate cell fate decisions. However, prolonged elevation or reduction in O-GlcNAc leads to a maladaptive response and is associated with pathologies such as hypertrophy and heart failure. In this review, we discuss the impact of O-GlcNAc in both acute and prolonged models of injury with a focus on the heart and biological mechanisms that underpin cell survival.
Keywords: ER stress; autophagy; cardioprotection; cellular stress response; chaperone; glycoprotein; heart failure; hypertrophy; integrated stress response.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
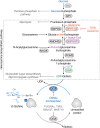
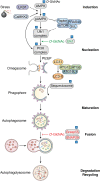
 ): AMPK (249), ULK1, Beclin1, and p62/sequestosome (56). Steps promoted and inhibited by O-GlcNAcylation are identified. AMPK, 5′-AMP-activated protein kinase; ATG, autophagy; CaMK II, calcium/calmodulin-dependent protein kinase kinase 2; DFCP1, double FYVE-containing protein 1; GRASP55, Golgi reassembly-stacking protein of 55 kDa; LC3, microtubule-associated proteins 1A/1B light chain 3B; LKB1, liver kinase B1; mTOR, mammalian target of rapamycin; PIP3, phosphatidylinositol-3,4,5-triphosphate; SNAP29, synaptosomal-associated protein 29; ULK1, Unc-51–like kinase 1; WIPI1, WD repeat domain phosphoinositide-interacting protein 1.
): AMPK (249), ULK1, Beclin1, and p62/sequestosome (56). Steps promoted and inhibited by O-GlcNAcylation are identified. AMPK, 5′-AMP-activated protein kinase; ATG, autophagy; CaMK II, calcium/calmodulin-dependent protein kinase kinase 2; DFCP1, double FYVE-containing protein 1; GRASP55, Golgi reassembly-stacking protein of 55 kDa; LC3, microtubule-associated proteins 1A/1B light chain 3B; LKB1, liver kinase B1; mTOR, mammalian target of rapamycin; PIP3, phosphatidylinositol-3,4,5-triphosphate; SNAP29, synaptosomal-associated protein 29; ULK1, Unc-51–like kinase 1; WIPI1, WD repeat domain phosphoinositide-interacting protein 1.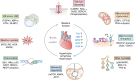
References
-
- Kültz D. Molecular and evolutionary basis of the cellular stress response. Physiology. 2005;67:225–257. - PubMed
-
- Kültz D. Evolution of cellular stress response mechanisms. J. Exp. Zoöl. Part A: Ecol. Integr. Physiol. 2020;333:359–378. - PubMed
-
- Divya S., Ravanan P. Cellular battle against endoplasmic reticulum stress and its adverse effect on health. Life Sci. 2023;323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

