Recent Developments in On-Demand Voiding Therapies
- PMID: 38641354
- PMCID: PMC11338280
- DOI: 10.1124/jpet.123.002073
Recent Developments in On-Demand Voiding Therapies
Erratum in
-
Corrigendum to "Recent Developments in On-Demand Voiding Therapies" [The Journal of Pharmacology and Experimental Therapeutics 390 (2024) 302-317].J Pharmacol Exp Ther. 2025 Sep;392(9):103675. doi: 10.1016/j.jpet.2025.103675. Epub 2025 Aug 18. J Pharmacol Exp Ther. 2025. PMID: 40834653 Free PMC article. No abstract available.
Abstract
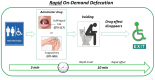
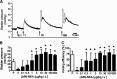
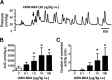
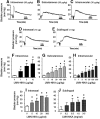
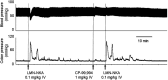
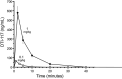
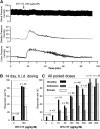
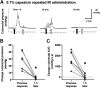
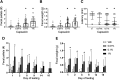
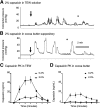
One cannot survive without regularly urinating and defecating. People with neurologic injury (spinal cord injury, traumatic brain injury, stroke) or disease (multiple sclerosis, Parkinson's disease, spina bifida) and many elderly are unable to voluntarily initiate voiding. The great majority of them require bladder catheters to void urine and "manual bowel programs" with digital rectal stimulation and manual extraction to void stool. Catheter-associated urinary tract infections frequently require hospitalization, whereas manual bowel programs are time consuming (1 to 2 hours) and stigmatizing and cause rectal pain and discomfort. Laxatives and enemas produce defecation, but onset and duration are unpredictable, prolonged, and difficult to control, which can produce involuntary defecation and fecal incontinence. Patients with spinal cord injury (SCI) consider recovery of bladder and bowel function a higher priority than recovery of walking. Bladder and bowel dysfunction are a top reason for institutionalization of elderly. Surveys indicate that convenience, rapid onset and short duration, reliability and predictability, and efficient voiding are priorities of SCI individuals. Despite the severe, unmet medical need, there is no literature regarding on-demand, rapid-onset, short-duration, drug-induced voiding therapies. This article provides in-depth discussion of recent discovery and development of two candidates for on-demand voiding therapies. The first, [Lys3,Gly8,-R-γ-lactam-Leu9]-NKA(3-10) (DTI-117), a neurokinin2 receptor agonist, induces both urination and defecation after systemic administration. The second, capsaicin (DTI-301), is a transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor agonist that induces defecation after intrarectal administration. The review also presents clinical studies of a combination drug therapy administered via iontophoresis and preclinical studies of neuromodulation devices that induce urination and defecation. SIGNIFICANCE STATEMENT: A safe and effective, on-demand, rapid-onset, short-duration, drug-induced, voiding therapy could eliminate or reduce need for bladder catheters, manual bowel programs, and colostomies in patient populations that are unable to voluntarily initiate voiding. People with spinal injury place more importance on restoring bladder and bowel control than restoring their ability to walk. This paradigm-changing therapy would reduce stigmatism and healthcare costs while increasing convenience and quality of life.
Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



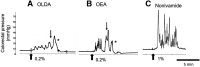
References
-
- Anderson KD (2004) Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21:1371–1383. - PubMed
-
- Ashburn TTThor KB (2004) . Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. - PubMed
-
- Brunton LL, Knollmann BC (2023) Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 14th ed. McGraw-Hill Education.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
