RBM22 regulates RNA polymerase II 5' pausing, elongation rate, and termination by coordinating 7SK-P-TEFb complex and SPT5
- PMID: 38641822
- PMCID: PMC11027413
- DOI: 10.1186/s13059-024-03242-6
RBM22 regulates RNA polymerase II 5' pausing, elongation rate, and termination by coordinating 7SK-P-TEFb complex and SPT5
Abstract
Background: Splicing factors are vital for the regulation of RNA splicing, but some have also been implicated in regulating transcription. The underlying molecular mechanisms of their involvement in transcriptional processes remain poorly understood.
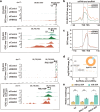
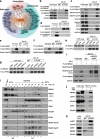
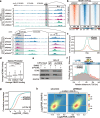
Results: Here, we describe a direct role of splicing factor RBM22 in coordinating multiple steps of RNA Polymerase II (RNAPII) transcription in human cells. The RBM22 protein widely occupies the RNAPII-transcribed gene locus in the nucleus. Loss of RBM22 promotes RNAPII pause release, reduces elongation velocity, and provokes transcriptional readthrough genome-wide, coupled with production of transcripts containing sequences from downstream of the gene. RBM22 preferentially binds to the hyperphosphorylated, transcriptionally engaged RNAPII and coordinates its dynamics by regulating the homeostasis of the 7SK-P-TEFb complex and the association between RNAPII and SPT5 at the chromatin level.
Conclusions: Our results uncover the multifaceted role of RBM22 in orchestrating the transcriptional program of RNAPII and provide evidence implicating a splicing factor in both RNAPII elongation kinetics and termination control.
Keywords: 5′ pausing; RBM22; RNA polymerase II; Transcription elongation; Transcription termination.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 2019YFA0802801/National Key Research and Development Program of China
- 32371356/National Natural Science Foundation of China
- 2042022dx0003/Fundamental Research Funds for the Central Universities
- ZNJC202009/the Translational Medicine and Multidisciplinary Research Project of Zhongnan Hospital of Wuhan University
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

