Efgartigimod and Ravulizumab for Treating Acetylcholine Receptor Auto-antibody-Positive (AChR-Ab+) Generalized Myasthenia Gravis: Indirect Treatment Comparison
- PMID: 38642198
- PMCID: PMC11133097
- DOI: 10.1007/s12325-024-02856-3
Efgartigimod and Ravulizumab for Treating Acetylcholine Receptor Auto-antibody-Positive (AChR-Ab+) Generalized Myasthenia Gravis: Indirect Treatment Comparison
Abstract
Introduction: Efgartigimod and ravulizumab, both approved for treating acetylcholine receptor auto-antibody-positive (AChR-Ab+) generalized myasthenia gravis (gMG), have not been directly compared. This paper assessed comparative effects of efgartigimod vs. ravulizumab for treating adults with AChR-Ab+ gMG using indirect treatment comparison methods.
Methods: The matching-adjusted indirect comparison used data from two randomized trials of adult men and women. The ADAPT (efgartigimod vs. placebo; individual patient data available) population was reweighted to match the CHAMPION (ravulizumab vs. placebo; index study; aggregate data available) population. The relative effect of efgartigimod versus placebo was estimated in this reweighted population and compared with the observed ravulizumab versus placebo effect to estimate the efgartigimod versus ravulizumab effect. The outcomes were Myasthenia Gravis Activities of Daily Living (MG-ADL), Quantitative Myasthenia Gravis (QMG), and Myasthenia Gravis Quality of Life 15-item-revised scale (MG-QoL15r) assessed as cumulative effect (area under the curve; AUC) over 26 weeks (primary) and change from baseline at 4 weeks and time of best response (week 4 for efgartigimod; week 26 for ravulizumab).
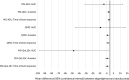
Results: For MG-QoL15r, efgartigimod had a statistically significant improvement compared with ravulizumab over 26 weeks [mean difference (95% confidence interval): - 52.6 (- 103.0, - 2.3)], at week 4 [- 4.0 (- 6.6, - 1.4)], and at time of best response [- 3.9 (- 6.5, - 1.3)]. Efgartigimod had a statistically significant improvement over ravulizumab in MG-ADL at week 4 [- 1.9 (- 3.3, - 0.5)] and at time of best response [- 1.4 (- 2.8, 0.0)] and in QMG at week 4 [- 3.2 (- 5.2, - 1.2)] and at time of best response [- 3.0 (- 5.0, - 1.0)]. For AUC over 26 weeks, improvements were not significantly different between efgartigimod and ravulizumab for MG-ADL [- 8.7 (- 36.1, 18.8)] and QMG [- 13.7 (- 50.3, 22.9)].
Conclusion: Efgartigimod may provide a faster and greater improvement over 26 weeks in quality of life than ravulizumab in adults with AChR-Ab+ gMG. Efgartigimod showed faster improvements in MG-ADL and QMG than ravulizumab.
Keywords: AChR-Ab+; Acetylcholine receptor auto-antibodies positive; Efgartigimod; Generalized myasthenia gravis; MG-ADL; MG-QoL15; QMG; Ravulizumab.
© 2024. The Author(s).
Conflict of interest statement
Cécile van Steen and Sergio Iannazzo were employees of argenx BV, who manufacture efgartigimod, when the manuscript was completed. Cécile van Steen has since changed affiliation to Biogen Netherlands BV. Celico, Spaepen, Bodicoat and de Francesco have received research funding from argenx BV. Hagenacker has received research support from Biogen, Novartis GeneTherapies, Roche and Sanofi Genzyme, speakers and consultant honoraria from Biogen, Hormosan, Roche, Alexion, Novartis, Roche, Sanofi-Genzyme, Alnylam and Argenx. Sven G. Meuth received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer HealthCare, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Alexion, Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. Tobias Ruck reports grants from German Ministry of Education, Science, Research and Technology; grants and personal fees from Sanofi-Genzyme; personal fees from Biogen and Novartis; personal fees and non-financial support from Merck Serono; personal fees from Roche, Teva, Alexion, argenx BV, UCB, and BMS. A Gordon Smith has received research support from Alexion, argenx BV, Janssen, Seismic, and UCB.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

