Marine sulfated glycans inhibit the interaction of heparin with S-protein of SARS-CoV-2 Omicron XBB variant
- PMID: 38642280
- PMCID: PMC12185386
- DOI: 10.1007/s10719-024-10150-1
Marine sulfated glycans inhibit the interaction of heparin with S-protein of SARS-CoV-2 Omicron XBB variant
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a worldwide COVID-19 pandemic, leading to 6.8 million deaths. Numerous variants have emerged since its outbreak, resulting in its significantly enhanced ability to spread among humans. As with many other viruses, SARS‑CoV‑2 utilizes heparan sulfate (HS) glycosaminoglycan (GAG) on the surface of host cells to facilitate viral attachment and initiate cellular entry through the ACE2 receptor. Therefore, interfering with virion-HS interactions represents a promising target to develop broad-spectrum antiviral therapeutics. Sulfated glycans derived from marine organisms have been proven to be exceptional reservoirs of naturally existing HS mimetics, which exhibit remarkable therapeutic properties encompassing antiviral/microbial, antitumor, anticoagulant, and anti-inflammatory activities. In the current study, the interactions between the receptor-binding domain (RBD) of S-protein of SARS-CoV-2 (both WT and XBB.1.5 variants) and heparin were applied to assess the inhibitory activity of 10 marine-sourced glycans including three sulfated fucans, three fucosylated chondroitin sulfates and two fucoidans derived from sea cucumbers, sea urchin and seaweed Saccharina japonica, respectively. The inhibitory activity of these marine derived sulfated glycans on the interactions between RBD of S-protein and heparin was evaluated using Surface Plasmon Resonance (SPR). The RBDs of S-proteins from both Omicrion XBB.1.5 and wild-type (WT) were found to bind to heparin, which is a highly sulfated form of HS. All the tested marine-sourced sulfated glycans exhibited strong inhibition of WT and XBB.1.5 S-protein binding to heparin. We believe the study on the molecular interactions between S-proteins and host cell glycosaminoglycans provides valuable insight for the development of marine-sourced, glycan-based inhibitors as potential anti-SARS-CoV-2 agents.
Keywords: Heparin; Marine sulfated glycans; Omicron XBB.1.5; SARS-CoV-2; SPR; Spike Protein.
© 2024. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Declarations
Figures


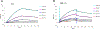

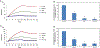
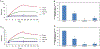
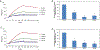
Similar articles
-
Disrupting SARS-CoV-2 Spike-ACE2 Interactions via Glycosaminoglycans in a Pseudoviral Study of Heparan Sulfate and Enoxaparin.Biomolecules. 2025 Jun 26;15(7):931. doi: 10.3390/biom15070931. Biomolecules. 2025. PMID: 40723804 Free PMC article.
-
Comprehensive N-glycosylation profiling of recombinant spike S1 protein from the wild-type SARS-CoV-2 and its variants.Front Immunol. 2025 Jul 16;16:1592142. doi: 10.3389/fimmu.2025.1592142. eCollection 2025. Front Immunol. 2025. PMID: 40755778 Free PMC article.
-
Conformational Dynamics and Binding Interactions of SARS-CoV-2 Spike Protein Variants: Omicron, XBB.1.9.2, and EG.5.J Chem Inf Model. 2025 Jul 28;65(14):7651-7667. doi: 10.1021/acs.jcim.5c00308. Epub 2025 Jul 11. J Chem Inf Model. 2025. PMID: 40643983 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
Cited by
-
A Toxoplasma gondii thioredoxin with cell adhesion and antioxidant function.Front Cell Infect Microbiol. 2024 Aug 15;14:1404120. doi: 10.3389/fcimb.2024.1404120. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39211799 Free PMC article.
-
Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity.Biomacromolecules. 2025 Aug 11;26(8):5169-5181. doi: 10.1021/acs.biomac.5c00576. Epub 2025 Jul 6. Biomacromolecules. 2025. PMID: 40619669 Free PMC article.
-
Applications of Surface Plasmon Resonance in Heparan Sulfate Interactome Research.Biomedicines. 2025 Jun 14;13(6):1471. doi: 10.3390/biomedicines13061471. Biomedicines. 2025. PMID: 40564190 Free PMC article. Review.
References
-
- Qu P, Faraone JN, Evans JP, Zheng Y-M, Carlin C, Anghelina M, Stevens P, Fernandez S, Jones D, Panchal AR, Saif LJ, Oltz EM, Zhang B, Zhou T, Xu K, Gumina RJ, Liu S-L: Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants. Cell. Rep. 42, 112443 (2023). 10.1016/j.celrep.2023.112443 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

