Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?
- PMID: 38642912
- PMCID: PMC11188544
- DOI: 10.1093/cei/uxae031
Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?
Abstract
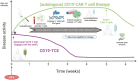
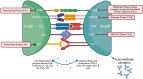
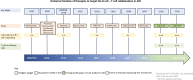
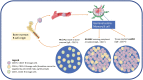
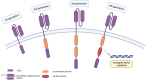
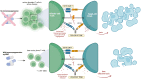
B and T cells collaborate to drive autoimmune disease (AID). Historically, B- and T-cell (B-T cell) co-interaction was targeted through different pathways such as alemtuzumab, abatacept, and dapirolizumab with variable impact on B-cell depletion (BCD), whereas the majority of patients with AID including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and organ transplantation benefit from targeted BCD with anti-CD20 monoclonal antibodies such as rituximab, ocrelizumab, or ofatumumab. Refractory AID is a significant problem for patients with incomplete BCD with a greater frequency of IgD-CD27+ switched memory B cells, CD19+CD20- B cells, and plasma cells that are not directly targeted by anti-CD20 antibodies, whereas most lymphoid tissue plasma cells express CD19. Furthermore, B-T-cell collaboration is predominant in lymphoid tissues and at sites of inflammation such as the joint and kidney, where BCD may be inefficient, due to limited access to key effector cells. In the treatment of cancer, chimeric antigen receptor (CAR) T-cell therapy and T-cell engagers (TCE) that recruit T cells to induce B-cell cytotoxicity have delivered promising results for anti-CD19 CAR T-cell therapies, the CD19 TCE blinatumomab and CD20 TCE such as mosunetuzumab, glofitamab, or epcoritamab. Limited evidence suggests that anti-CD19 CAR T-cell therapy may be effective in managing refractory AID whereas we await evaluation of TCE for use in non-oncological indications. Therefore, here, we discuss the potential mechanistic advantages of novel therapies that rely on T cells as effector cells to disrupt B-T-cell collaboration toward overcoming rituximab-resistant AID.
Keywords: CAR T-cell therapy; T-cell engagers; rheumatoid arthritis; rituximab; systemic lupus erythematosus.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
None declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

